Electronic Submission Database (eIRB)
The NASA IRB uses an electronic submission system for the review of human research studies called eIRB. The site contains information that is accessible to the public, such as meeting dates and standard operating procedures. One must have a registered account in order to submit or alter studies.
Visit the eIRB about Electronic Submission Database (eIRB)
Follow the Submission through the eIRB
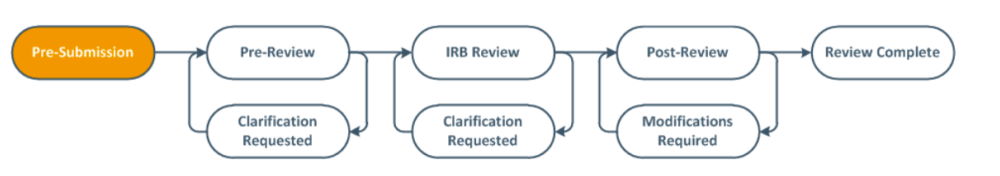
The eIRB system uses “states” to classify where a submission is in the review process. The diagram below demonstrates the workflow a submission will undergo, from the “Pre-Submission” state to “Review Complete”.
The following table defines some possible study states a submission could be in during the course of its review.
| Pre-Submission | The application is under preparation by the Principal Investigator (PI) or submission preparer. |
| Clarification Requested | The IRB Coordinator has requested changes or clarification. The PI and Primary Contact will receive an e-mail notification advising them of the request. |
| Pre-Review Completed | The submission awaits assignment to a Designated IRB member for review. |
| Non-Committee Review | The submission is currently being reviewed by an IRB member. |
| Committee Review | The submission awaits assignment to the Convened IRB for review or the submission has been assigned to an IRB meeting and is being reviewed by the IRB members. |
| Post Review | The IRB letter advising the PI of the Board’s or IRB member’s decision is being prepared. |
| Review Complete | Review of the submission is complete and a letter has been sent. |
Submission Methods
Below are several categories of submissions that are used in the eIRB. Click on the categories to become familiar with them and read the attached document to learn how to submit them in the eIRB.
New Study
Before submitting a new study, determine the Principal Investigator and identify the study team. All members of the study will have completed human subjects protections training and conflict of interest requirements. Develop the study protocol, informed consent, and gather supporting documents such as advertisements and questionnaires/surveys. Review all documents thoroughly for consistency before submission.

Modifications to Approved Studies
Investigators are responsible for ongoing requirements in the conduct of approved research. This includes obtaining prior approval from the NASA IRB for any modifications of the previously approved research before implementing the proposed modification.

Continuing Review/Continuing Review with Modifications
Continuing Review (CR) is required for all studies determined to be greater than minimal risk and FDA-regulated research; it may be required for minimal risk studies. When required, CR occurs at least annually until all research-related activities are completed. Conflict of interest disclosures must be updated at least annually for all key personnel.

Reportable New Information (RNI)
If investigators change the research in order to eliminate apparent immediate hazards without prior IRB approval or if a hazard occurs, it would need to be reported promptly. The NASA IRB uses a special submission in the eIRB for researchers to submit such events.

Study Closure
All IRB approved research protocols require a study closure when research activities are completed. Research activities include, but not limited to, participant enrollment, recruitment, data collection, storage of identifiable data, and sharing identifiable data. The study cannot be closed if any research activities are occurring and NASA IRB approval must be maintained through a continuing review, where appropriate.

Reliance Acknowledgment and Individual Investigator Acknowledgment
The NASA IRB can serve as the IRB of record or cede review to an external IRB, depending on how the research is being conducted. It holds a Federal-wide Assurance (FWA), FWA00019876, that can help facilitate cooperative research between institutions.
If a collaborating investigator is not affiliated with an institution or organization holding an FWA, an Individual Investigator Acknowledgement can be obtained.