Eduardo A.C. Almeida
Senior Scientist
Phone: (650) 604-1772
Email: E.Almeida@nasa.gov
Affiliation: Space Biosciences Research Branch
Biography
- Co-Director NASA Ames (2001-Present)
- Assistant Adjunct Professor, University of California, San Francisco, Department of Stomatology/Tissue and Cell Biology 2001-2005
- NASA Spaceflight Principal Investigator – Newt Tail Regeneration US/Russian Foton M2 and Foton M3 (2004-2008)
- NASA Spaceflight Principal Investigator – Space Tissue Loss (STL-1 STS-131, STL2 STS 135 (2009-2011)
- NASA Spaceflight Principal Investigator – US/Russian Bion-M1 Mouse BSP (STS-131, STS-133, STS 135, Bion-M1 (2007-2013)
- NASA Project Scientist International Space Station Bioculture System Flight Hardware Development and Validation (2012-2020)
- NASA Project Scientist International Space Station WetLab-2 qPCR System Flight Hardware Development and Validation (2012-2017)
- Code SC Science Representative for Space Biosciences Collaborative Building (N-288) Planning and Design (2013-2020)
- NASA Space Biology Discipline Scientist for Cell and Molecular Biology (2016-Present)
- NASA Spaceflight Principal Investigator – Rodent Research 10 on SPX-21/ISS -The role of p21/CDKN1a Pathway in Microgravity-induced Bone Tissue Regenerative Arrest – A Spaceflight Study of Transgenic p21/CDKN1a Null Mice (2014-2024)
Education
Postdoctoral, University of California, San Francisco
Postdoctoral, University of Virginia
Ph.D. in Zoology, University of California, Davis
M.S. in Biology, University of San Francisco
B.S. in Biology, University of San Francisco
Research Interests
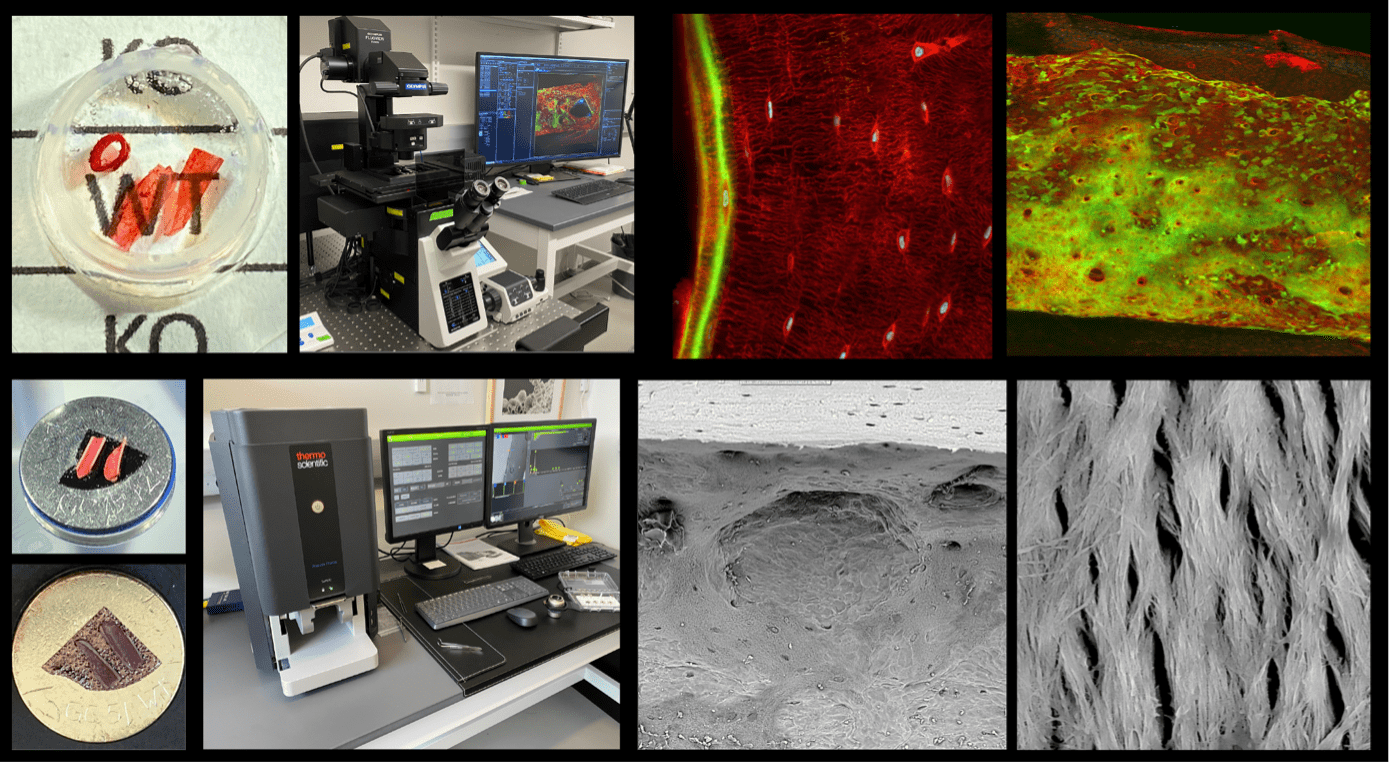
The research of Dr. Almeida’s group in the NASA Ames Bone and Cell Signaling Lab focuses on the effects of microgravity mechanical unloading on stem-cell-based tissue regeneration in space. Specifically, the group is testing the broad hypothesis that mechanical load from Earth’s gravity at 1 g is required for normal adult stem cell proliferation and differentiation during tissue regeneration and homeostasis. Research from the lab has already provided significant evidence supporting the gravity tissue regeneration hypothesis in various biological models, including embryonic stem cells (STS-131 and STS-135), newt regenerating tails (Foton M-2 and Foton M-3), mouse bone (Bion M-1, STS-131, STS-133, STS-135, SPX21), and led to the identification of the cell cycle inhibitor p21/CDKN1a as a candidate molecular mediator mechanism of bone marrow mesenchymal and hematopoietic lineage tissue regenerative arrest in microgravity, recognized with the NASA 2017 Exceptional Scientific Achievement Medal (NASA ISS Office, Houston, TX). This hypothesis was tested in the NASA Rodent Research 10 spaceflight experiment successfully conducted on ISS/SPX-21 with p21/Cdkn1a null mice. https://www.nasa.gov/ames/research/space-biosciences/rodent-research-10-spacex-21 Our technical approaches for ongoing studies include using confocal microscopy of optically cleared bone to measure mineral appositional growth and mineralized calcein-labeled surface during spaceflight, as well as correlating mineralization with bone surface by osteoclasts imaged with Scanning Electron Microscopy (Figure 1).
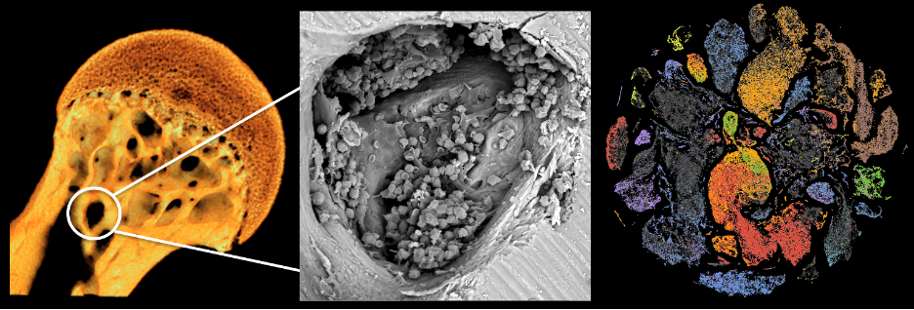
Our studies also included structural analyses of bone morphometric parameters using x-ray microcomputed tomography (micro-CT) (Figure 2, left panel) and single cell genomic analysis of bone marrow mesenchymal and hematopoietic stem cell progenitor lineages in microgravity and in ground altered condition of mechanical loading and unloading (Figure 2, center and right panels).
In addition to basic research, Dr. Almeida has also been NASA Project Scientist and Space Biology Discipline Scientist, leading efforts to develop new cell and molecular biology techniques and tools for use in microgravity on the International Space Station, such as the WetLab2 suite of Molecular Biology qRT PCR capability (SPX-9), and Bioculture System for on-orbit Cell Science investigations flown on SPX-13. Dr. Almeida’s NASA service also included significant contributions to the shared open science collaborative concept and design for the new ARC Space Bioscience Collaborative Laboratory building N288, recognized in 2020 with the NASA Exceptional Achievement Medal (NASA ARC). Previous significant contributions to science at UCSF, include the identification of extracellular matrix/integrin cell survival signaling mechanisms that suppress p53 in the absence of serum growth factors, and characterizing the mouse egg alpha 6 integrin receptor activity, for the ADAMS family sperm ligand Fertilin in mammalian fertilization.
Collaborators and Associates
NASA Ames Research Center: Yasaman Shirazi-Fard, Ph.D. (Laboratory Co-Director, RR10 Co-I); Mark Ditzler, Ph.D.; Ruth K. Globus, Ph.D. Ret.; Emily Holton Ph.D., Lab Founder, Ret. Emeritus Member.
BlueMarble Space Institute of Sciences at NASA Ames Research Center, Kira Reinecker, Ph.D.; Kieran Brown; Rukmani Cahill.
Rensselaer Polytechnic Institute: Prof. Elizabeth Blaber, Ph.D.; (RR10 Co-I), Angela Kubic, Rukmani Cahill.
Embry Riddle Aeronautical University: Prof. Cassandra M. Juran, Ph.D., (RR10 Co-I).
University of California Berkeley: Prof. Grace D. O’Connell, Ph.D., PI Berkeley; Shiyin Lim; Emily Lindberg, (RR10 Collaborators).
University College London: Keith Siew, Ph.D. Department of Renal Medicine (RR10 Collaborator).
University of Kansas Medical Center: Prof. Lane Christenson, Ph.D, Department of Molecular and Integrative Physiology, (RR10 Collaborator).
Select Publications
- Cosmic Kidney Disease: The Effects of Spaceflight and Galactic Cosmic Radiation on Renal Structure and Function. Siew, K. et al. Preprint Research Square (2023)
- Differential Single Cell Responses of Embryonic Stem Cells Versus Embryoid Bodies to Gravity Mechanostimulation. Stem Cell and Development Juran, C.M., Zvirblyte, J., and Almeida, E.A.C., (2022) Stem Cells and Development, 31.13-14 (2022): 346-356
- Spaceflight Modulates the Expression of Key Oxidative Stress and Cell Cycle Related Genes in Heart. Kumar, A., Tahimic, C., Almeida, E., & Globus, R. K. (2021), International journal of molecular sciences, 22(16), 9088
- Cdkn1a deletion or suppression by cyclic stretch enhance the osteogenic potential of bone marrow mesenchymal stem cell-derived cultures. Juran, C.M., Blaber, E.A., Zvirblyte, J., and Almeida, E.A.C. (2021), Stem Cell Research, 102513.
- Evaluating the cytocompatibility and differentiation of bone progenitors on electrospun zein scaffolds J. Cardenas-Turner, G. Collins, E. A. Blaber, E. A. C. Almeida, T. L. Arinzeh, J. Tissue Eng. Regen Med 14:173-185, 2020
- Exposure to microgravity for 30 days onboard Bion M1 caused muscle atrophy and decreased regeneration in murine femoral quadriceps Radugina, E.A., Almeida, E.A.C., Blaber, E.A., Poplinskya, V.A., Markitantova, Y.V., Grigoryan, E.N. (2018), Life Sciences in Space Research, Vol 16, pg. 18-25
- Microgravity Validation of a Novel System for RNA Isolation and Multiplex Quantitative Real Time PCR Analysis of Gene Expression on the International Space Station. Macarena Parra, Eduardo A. C. Almeida et al.,(2017) PLOS ONE PONE-D-17-00722
- The effect of hypergravity on the lens, cornea and tail regeneration in Urodela, E.N. Grigoryan, N. Dvorochkin, V.A. Poplinskaya, R. Yousuf, E.A. Radugina, E.A. Almeida, Acta Astronautica, Volume 138, (2017), pg. 423-433
- Microgravity Reduces the Differentiation and Regenerative Potential of Embryonic Stem Cells. Blaber, E.A., Finkelstein, H., Dvorochkin, N., Sato, K., Yousuf, R., Burns, B.P. Globus, R.K., Almeida, E.A.C. (2015) Stem Cells and Development, Volume 24, Number 22, 2015
- Stem Cell Health and Tissue Regeneration in Microgravity. Blaber, E.A., Sato, K., Almeida, E.A.C. (2014) Stem Cells and Development, 23 Suppl. 1: 73-8
- Mechanical Unloading of Bone in Microgravity Reduces Mesenchymal and Hematopoietic Stem Cell-Mediated Tissue Regeneration. Blaber E.A., Dvorochkin, N., Torres, M.L., Yousuf, R., Burns, B.P., Globus, R.K., Almeida, E.A.C. (2014) Stem Cell Research, Vol 13(2): 181-201
- Microgravity Induces Pelvic Bone Loss through Osteoclastic Activity, Osteocytic Osteolysis, and Osteoblastic Cell Cycle Inhibition by CDKN1a/p21. Elizabeth A Blaber, Natalya Dvorochkin, Chialing Lee, Joshua S Alwood, Rukhsana Yousuf, Piero Pianetta, Ruth K Globus, Brendan P Burns, Eduardo A C Almeida, (2013) PLoS ONE), 8(4):e61372.
- Nanoscale X-ray microscopic imaging of mammalian mineralized tissue. Joy C Andrews, Eduardo Almeida,Marjolein C H van der Meulen, Joshua S Alwood, Chialing Lee, Yijin Liu, Jie Chen, Florian Meirer, Michael Feser, Jeff Gelb, Juana Rudati, Andrei Tkachuk, Wenbing Yun, Piero Pianetta, Microscopy and Microanalysis,(2010) 16(3):327-36.
- Matrix Survival Signaling: from Fibronectin Via Focal Adhesion Kinase to c-Jun NH2-Terminal Kinase. E. A. C. Almeida, D. Ilic, Q. Han, C. R. Hauck, F. Jin, H. Kawakatsu, D. D. Schlaepfer, C. H. Damsky, The Journal of Cell Biology, (2000) 149, 741-754.
- Extracellular Matrix Survival Signals Transduced by Focal Adhesion Kinase Suppress p53-Mediated Apoptosis. D.Ilic, E. A. C. Almeida, D. D. Schlaepfer, C. H. Damsky, The Journal of Cell Biology, (1998) 143, 547-560
- ADAMs and Cell Fusion. A.-P. J. Huovila, E. A. C. Almeida and J. M. White, Current Opinion in Cell Biology, (1996) 8, 692-699.
- Mouse Egg Integrin α6β1 Functions as a Sperm Receptor. E. A. C. Almeida, A. -P. J. Huovila, A. E. Sutherland,L. E. Stephens, P. G. Calarco, L. M. Shaw, A. M. Mercurio, A. Sonnenberg, P. Primakoff, D. G. Myles, and J. M. White, Cell, (1995) 81,1095-1104.
Awards
- NASA 2014 Exceptional Service Medal (NASA Ames Research Center, Silicon Valley, CA)
- NASA 2017 Exceptional Scientific Achievement Medal (NASA ISS Office, Houston, TX)
- NASA 2020 Exceptional Achievement Medal (NASA ARC, Silicon Valley, CA)