Micro-2 (STS-132)
Gravitational Effects on Biofilm Formation During Space Flight
Purpose: Understand the effects of microgravity on the growth, cellular physiology, and cell-cell interactions in microbial biofilms
Specimens: Pseudomonas aeruginosa and Staphylococcus aureus
Experiment Overview
There is an urgent need to understand the effects of microgravity on the growth, cellular physiology, and cell-cell interactions in microbial biofilms. This information can then be used to curtail harmful activities of microbial consortia thriving as biofilms on the International Space Station and for the long-term success of human space exploration. Bacterial biofilms were abundant on the Mir space station and were responsible for increasing corrosion and blocking a water purification system. Health and safety hazards linked to the development of biofilms are also of great concern, including increased rates of infection due to the enhanced survival of organisms within biofilms and increased resilience of biofilm populations to antimicrobial compounds.
The Micro-2 experiment studied how gravity alters biofilm formation with the goal of developing new strategies to reduce their impact on the operation of spacecrafts and the health of their crew.
Payload Overview
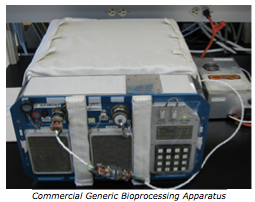
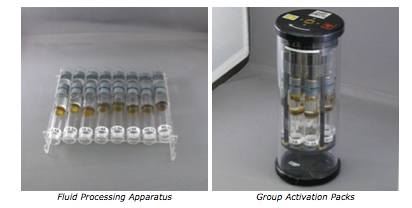
The Micro-2 experiment utilized BioServe’s flight certified hardware: Group Activation Packs (GAPs) stored in a Commercial Generic Bioprocessing Apparatus (CGBA). The CGBA is a flight certified incubator capable of controlling the temperature between 4°C and 37°C and can hold up to 16 GAPs. Each GAP holds eight Fluid Processing Apparatus (FPA) inserts. The FPA is composed of a glass barrel divided into three chambers that are separated from one another by rubber septa. Each FPA contained growth medium with membranes in chamber A, a microbial culture suspended in stasis medium in chamber B, and a termination reagent in chamber C.
Payload Overview
Launch & Return: STS-132 / ULF-4 Sortie Flight
Launched on May 14, 2010
Space shuttle Atlantis
Hardware:
- 32 Group Activation Pack (GAP) will be utilized with 1 Commercial Generic Bioprocessing Apparatus (CGBA)
- Middeck Locker
- 16 Flight GAPs & 16 Ground GAPs
- 8 Fluid Processing Apparatus (FPA) per GAP
Post-Flight
Dr. Collins and her team indicated that they are extremely happy with the quality of the samples received from the Micro-2 experiment on STS-132. Once the analysis is complete, the team plans to disseminate their results to the scientific community.
Project Team
Principal Investigator – Cynthia H. Collins, Ph.D., Rensselaer Polytechnic Institute, Troy, New York
Co-Investigator – Jonathan S. Dordick, Ph.D., Rensselaer Polytechnic Institute, Troy, New York
Co-Investigator – Joel L. Plawsky, Sc.D., Rensselaer Polytechnic Institute, Troy, New York
Project Manager – Tom Luzod, NASA, Ames Research Center
Project Scientist – Macarena Parra, Ph. D, Lockheed Martin, Ames Research Center
Hardware and Integration – BioServe Space Technologies, University of Colorado, Boulder
Louis Stodieck, Ph.D
Mark Rupert
Stephanie Countryman
Emily Pilinski
For more information, see the Space Station Research Explorer Micro-2 mission.