In situ Carbon Evolution Experiments (ICEE)
Members: Andrew Mattioda
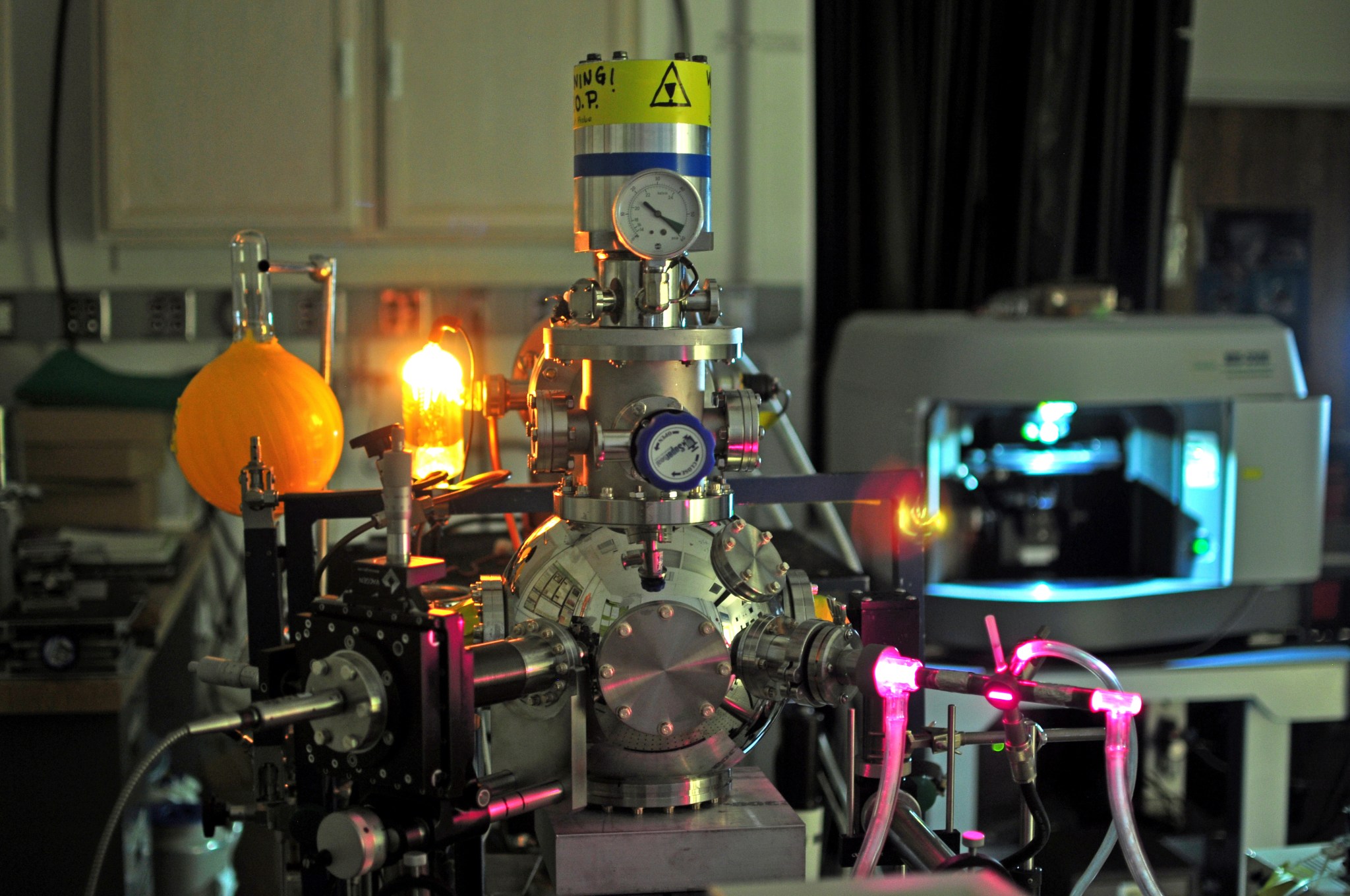
The ICEE facility is a unique NASA resource, with the ability to process samples via UV and electron radiation while monitoring them with IR, Raman, and Mass Spectroscopy.
HV Chamber Capabilities:
The HV chamber (10-9 torr) contains an IR transparent window, mounted onto the tip of a rotatable Helium cryo-cooler, capable of temperatures ranging from 15 to 300 K. Gas and solid sample deposition ports are located adjacent to each other in the HV chamber. The sample window can be positioned to face one or both deposition ports permitting the creation of mixed materials on the sample window.
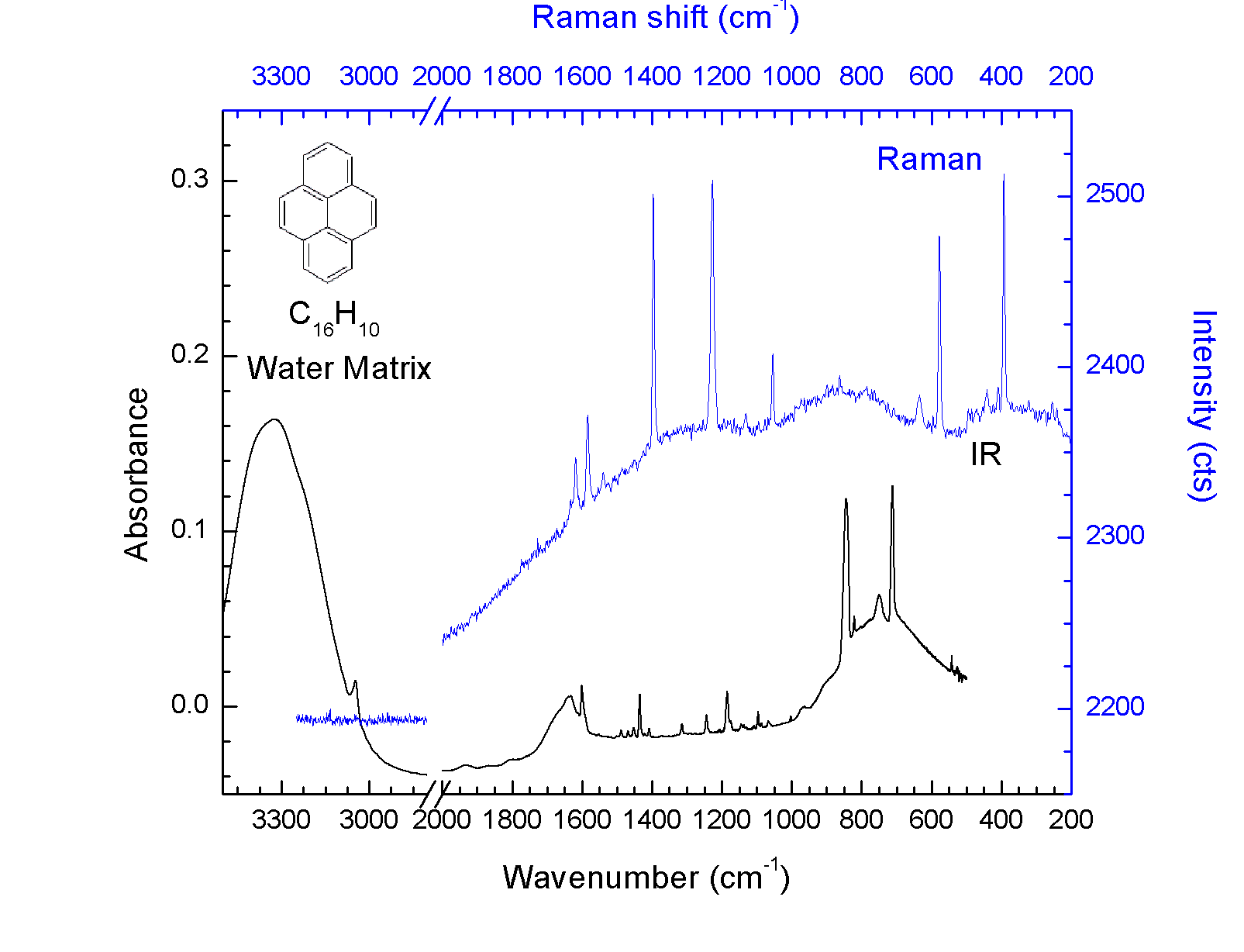
Three analytical instruments are available for in-situ sample analysis. A mass spectrometer, capable of analyzing the gas-phase molecules out to 300 u (atomic mass units). An FTIR spectrometer, permitting IR transmission measurements from ~600 nm to over 50 um, covering the UV, visible and IR spectral ranges. Fiber-optic probes (785 and 405 nm excitation wavelengths) permit the acquisition of in-situ Raman spectra.
The HV chamber contains two radiation sources, a high-energy electron gun, and a flowing H2 discharge lamp. The electron gun is capable of producing electrons from 1 – 100 keV, while the H2 microwave discharge lamp produces Lyman α photons (121.6 nm, 10.2 eV) as well as broad continuum emission. Samples can be positioned to face one or both radiation sources.
Raman Microscopic capabilities:
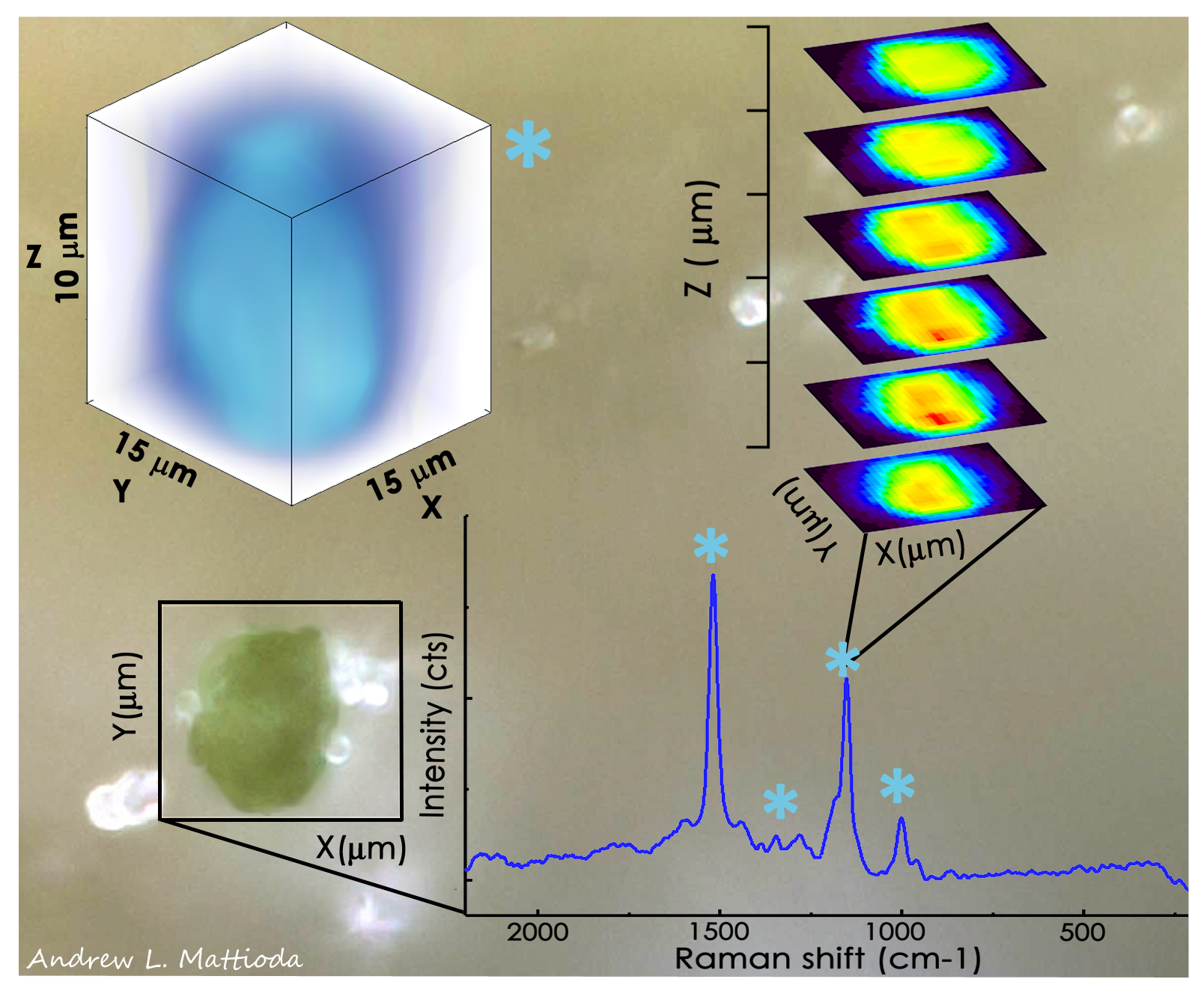
The Raman system is equipped with an auto microscope stage and 785, 532 and 405 nm excitation lasers permitting analysis of biological and thin-film sample analysis external to the HV chamber. The Raman microscope is equipped with a 5x, 20x, 50x and 100x objective lenses. The microscope is capable of spectroscopically mapping samples in 3D.
Raman and IR spectra are archived in online databases ensuring public access of the data to the scientific community and a legacy value to NASA.
DRIFTS capabilities:
DRIFTS (Diffuse Reflectance Infrared Fourier Transform Spectroscopy) is an infrared spectroscopy technique used to collect the reflected or scattered infrared light from the rough surface of a bulk powdered (e.g. regolith, mineral, dust) sample. The DRIFTS system is a Harrick Praying Mantis DRIFTS accessory fitted with a low temperature (~77 K), vacuum cell (~10-7 torr), which has been modified to accommodate an H2 microwave discharge lamp. Thus powdered samples can be irradiated with Lyman α photons (121.6 nm, 10.2 eV) as well as broad continuum emission. Similarly, powdered samples can be exposed, in-situ, to gases of choice using one of the three available inlet ports.
You can download the ICEE brochure here.