This article is from the 2020 NESC Technical Update.
One goal of the recent NESC assessment, Transient Combustion Modeling for Hypergolic Engines, was to identify and characterize the early reactions that occur between monomethylhydrazine (MMH) fuel and dinitrogen tetroxide (NTO) oxidizer in the liquid and gas phases to improve modeling for liquid-fueled space propulsion system hypergolic propellant engines. Drs. Tim Pourpoint and Hilkka Kenttämaa of Purdue University were asked to perform experiments to support the effort.
Identification of Reaction Products
Identifying the first products formed upon interactions of NTO and MMH requires an analytical technique capable of quickly and unambiguously providing elemental composition and structural information for the products. A combination of low- and high-resolution tandem mass spectrometry was chosen for this task. This technique requires the products to be converted into gas-phase ions before analysis.
The initial products formed upon liquid- and gas-phase hypergolic reactions may react immediately with other liquids or gases that form in the mixture. Because the reactions cannot be halted to collect the first species generated, evaporation and ionization (if necessary) must occur at the moment the products form to ensure that the correct species are being analyzed. Based on this condition, the team selected laser desorption/ionization (LDI) as the most promising technique due to its speed. The current state of laser technology enables laser pulse lengths on the order of nanoseconds, much shorter than the expected time scale of the reactions of interest.
LDI has been successfully used by researchers with a 355 nm laser to evaporate and ionize solid aromatic compounds1, proteins2,3, and polymers4. Since MMH and NTO are relatively small molecules, have different structures compared to the types of samples discussed in the literature, and have largely unknown early reaction products, the energy of the photons and the laser power (density of photons) required for LDI of their products were unknown.
Purdue Test Facility
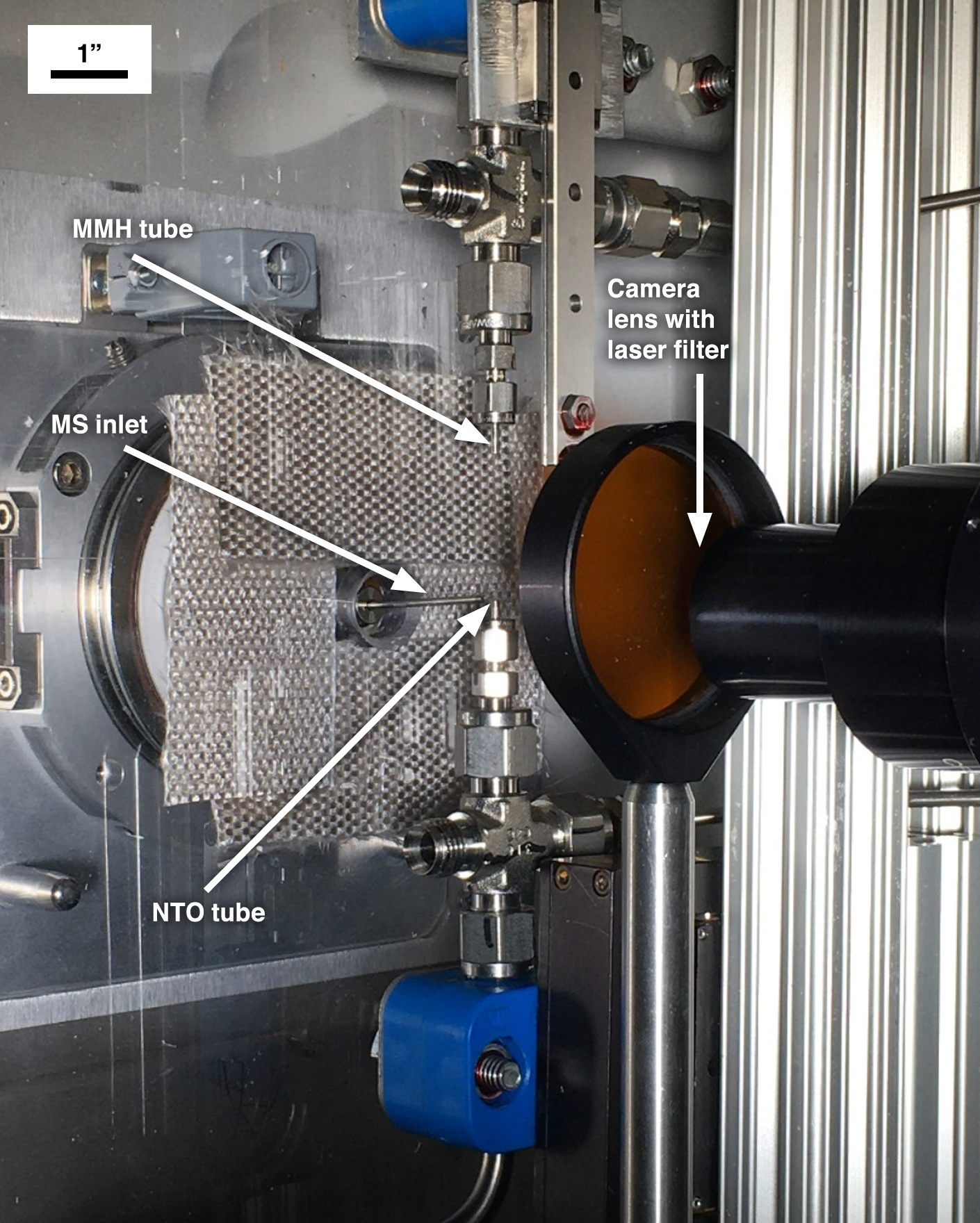
To conduct the investigation of the liquid phase and early gas phase reactions of MMH with NTO, the Purdue team designed an apparatus that brought approximately 3 µl drops of MMH and NTO into contact with each other in a highly repeatable manner, synchronized with the LDI technique, and under controlled conditions. The small liquid volumes made the experiment easier to control and improved safety. Figure 1 shows the final drop-on-drop experimental apparatus installed in a mobile fume hood. The NTO drop was placed into the bottom tube as opposed to MMH due to its low surface tension. The MMH drop was then moved down to touch the NTO drop by using an actuator with a maximum actuation speed of 14 inch/second and spatial resolution of 1 µm. This high actuator speed was chosen to minimize interactions between NTO and MMH vapors before the drops contacted one another.
MMH/NTO Drop-on-Drop Testing
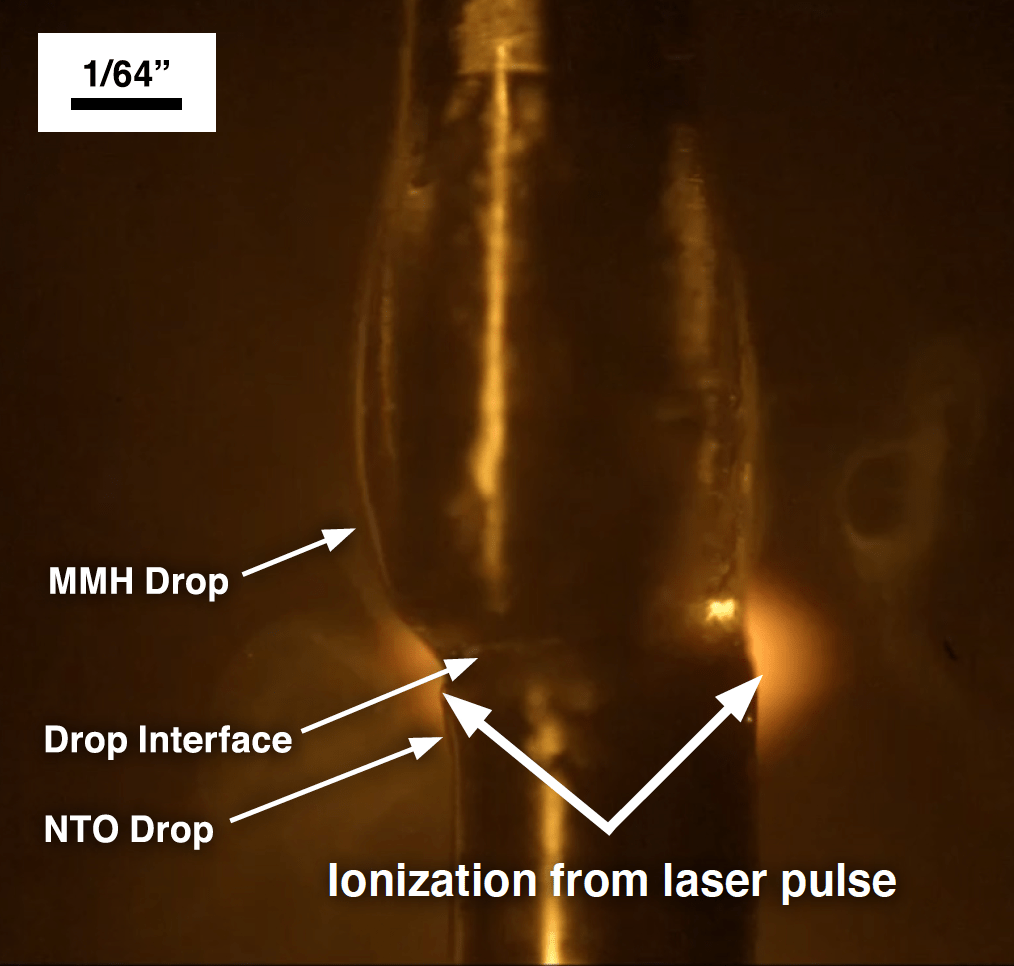
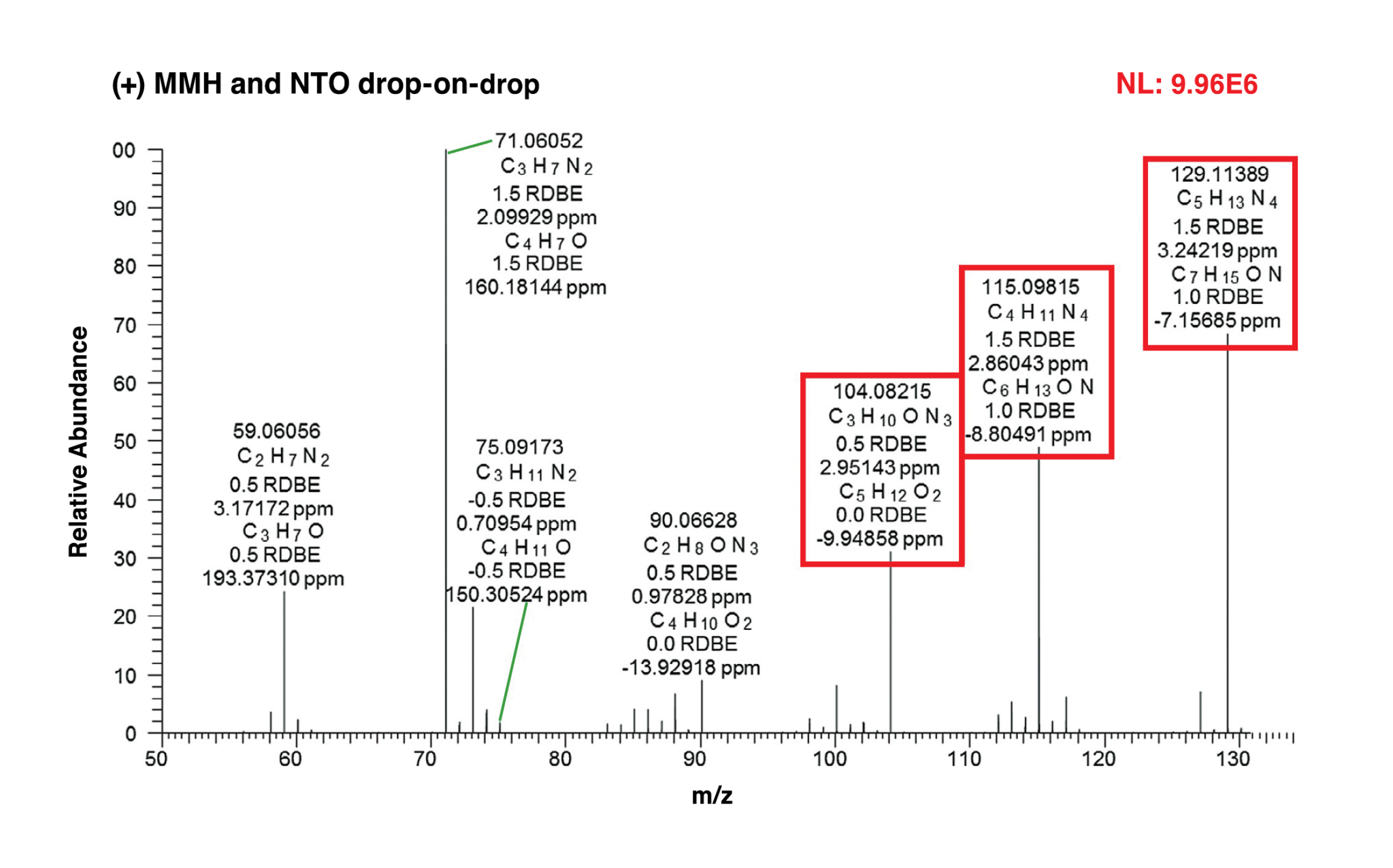
Prior to each experiment, the laser was allowed to warm up while the laser beam was blocked from entering the test area by a beam shutter. With the laser ready, the MMH drop was brought down and into contact with the NTO drop. Simultaneously, the data acquisition system sent a signal to the mass spectrometer to begin data acquisition. Shortly after triggering the mass spectrometer, the system sent a signal to open the beam shutter and allow a single laser pulse to pass next to the reaction just as the mass spectrometer began detecting ions. Figure 2 shows a still photo of the laser pulse hitting the area between the touching droplets and the mass spectrometer inlet during a test sequence. Evidence of the laser pulse is clearly visible because of the ionized gases created as the laser beam passes through the area. The orange coloration was caused by the laser filter used to protect the camera. Figure 3 shows a high-resolution mass spectrum measured for the MMH/NTO liquid reaction products showing the measured elemental compositions of the ions and proposed structures for some of the ions. Additional results demonstrated that the liquid-phase reactions of MMH and NTO readily produce large amounts of ions in the absence of any ionization method (i.e., LDI), which can be detected by the mass spectrometer. Aside from the ionic compounds produced, the neutral intermediates cannot be detected without LDI, which will be part of future experimentation.
Interestingly, while many positively charged ions were observed, only a few negatively charged ions, the most abundant corresponding to nitrate, were detected. These conclusions are in agreement with the nature of the highly energetic hypergolic reactions, as ions are much more reactive than neutral molecules in the gas phase. The results of the experiments conducted by the NESC assessment team will augment modeling capabilities with the objective of improving combustion instability predictions for existing and future hypergolic propellant engines. For more information, contact Dr. Daniel J. Dorney, daniel.j.dorney@nasa.gov.
References:
- Dotter, R. N.; Smith, C. H.; Young, M. K.; Kelly, P. B.; Jones, A. D.; McCauley, E. M.; Chang, D. P. Y. Laser Desorption/Ionization Time-of-Flight Mass Spectrometry of Nitrated Polycyclic Aromatic Hydrocarbons. Anal. Chem. 1996, 68 (14), 2319–2324.https://doi.org/10.1021/ac951132r
- Kawasaki, H.; Akira, T.; Watanabe, T.; Nozaki, K.; Yonezawa, T.; Arakawa, R. Sulfonate Group-Modified FePtCu Nanoparticles as a Selective Probe for LDI-MS Analysis of Oligopeptides from a Peptide Mixture and Human Serum Proteins. Anal Bioanal Chem 2009, 395 (5), 1423. https://doi.org/10.1007/s00216-009-3122-0
- Karas, M.; Ingendoh, A.; Bahr, U.; Hillenkamp, F. Ultraviolet–Laser Desorption/Ionization Mass Spectrometry of Femtomolar Amounts of Large Proteins. Biomedical & Environmental Mass Spectrometry 1989, 18 (9), 841–843. https://doi.org/10.1002/bms.1200180931
- Gołda-Cępa, M.; Aminlashgari, N.; Hakkarainen, M.; Engvall, K.; Kotarba, A. LDI-MS Examination of Oxygen Plasma Modified Polymer for Designing Tailored Implant Biointerfaces. RSC Adv. 2014, 4 (50), 26240–26243. https://doi.org/10.1039/C4RA02656J