Hello, George. We appreciate you setting aside a little bit of time for a conversation with us. We generally start by asking about your childhood, where you’re from, your family at that time, and if there was anything about your young years, or at what point did you start getting interested in science as a potential career?
OK, well childhood-wise I was born in the boondocks of Mississippi, as they say. Luckily, we were down there most of our very young years, my brother and I, and then we went to northern cities, back-and-forth. I went to high school in Detroit and honestly, there wasn’t a lot of science offered. Anyway, it wasn’t until I went to college at Western Michigan University, after two or three years that I discovered chemistry. Like probably everyone in undergrad, I would think of med school and what that would be like. And advisers said, “Well then you would have to take chemistry and biology”. I took, I think, both courses the same semester. In biology, after a week or so, they showed us these little critters that we would either have to dissect or kill, or both, and that was it for biology and medicine – I dropped the class right away! There went my medical career down the drain in one week! (laughs). But I really liked the chemistry. I said, “Wow, what is this?” Chemistry! Unfortunately, at that point, I then had to rush through a chem major and math minor. Anyway, chemistry has been my focus to this day.
Wow, that’s an interesting story. When you said you were born down in Mississippi, were you in kind of a rural setting or more in the city? And then what was it that moved your family up to Detroit?
Well, to Cleveland first and then back to Mississippi. It was a rural place that you’ve never heard of, but it was 30 minutes south of Mississippi State University in Starkville, so that’s the only place I can relate it to. My mother was just trying to find a better life by moving.
The reason I ask is that some of our researchers have talked about when they were little and were exposed to nature or the sky or being out in the woods and seeing the stars or the clouds, for example, and that’s what got them interested in those things.
Well, we were on a farm and I do remember standing outside at night viewing the stars. If you want to see dark skies go to rural Mississippi and look up: it was amazing to look at. I had no concept at that point of being an astronomer or anything scientific, but I guess it was always in the back of my mind. When I went to college I had to major in something, one major was communications. I don’t know if you remember Super 8 films? Well, I made a few of those but there was no subject that grabbed me yet at that point.
With a degree in chemistry, and then you had to decide to go to grad school so did you take on graduate work in chemistry? I guess I’m trying to navigate to your current interest in the chemical evidence of life in meteorites if I understand correctly? How did that journey happen?
Yeah, I’ll try to do it quickly. So after undergrad, bachelor’s degree in chemistry, my first goal was to go someplace warm because Kalamazoo, Michigan has to be the coldest place on earth! The Russians have nothing on cold compared to Kalamazoo! (laughs)
OK, so that got you to Arizona?
Well, first Texas. I got a reply letter from Baylor College of Medicine for a position there and I ended up deciding that wasn’t what I wanted. So the Texas Medical Center in Houston, I don’t know if you’ve ever been there, but there is a huge medical center there, they have the money, and there was the UT Medical School right next door, so I ended up as a lab tech to a cell biologist there. I didn’t go to grad school right away, I wanted to get a little more physical energy back so I took some graduate courses down there, and then a few years later I applied to different grad schools and Arizona State wrote back and sounded pretty good so that’s how I ended up at Arizona State with chemistry under Professor John Cronin. To your question of getting interested in this organic space chemistry field, he was a leader at that time in this business of the organic analysis of meteorites. The ones he analyzed (“carbonaceous” meteorites) are from asteroids that are 4.6 billion years old, so their organic compounds are the first organic molecules in the solar system, so that was kind of fascinating. And he had such a good reputation, it was easy to get into that business. I started the analysis and found organic phosphorus compounds and a few other things and ended up as a postdoc with Sherwood Chang here at Ames: Sherwood is retired now and still doing well. I have basically continued the same work, analysis, but also continue to try and find new organic material. That’s how I got to the finding of sugar derivatives in meteorites. The work wasn’t necessarily looking for life but looking for compounds that could have led to life, and that’s one of NASA’s core goals – understanding the universe’s chemistry that led to the first organisms. So that’s how I ended up doing what I’m doing.
OK, so your connection to Ames was initially as a postdoc with Sherwood?
Right. He of course knew John Cronin. They were in the same business, astrobiology before it was called astrobiology.
So you came here and then I guess after your postdoc, I don’t know how long after, you were able to get on as a civil servant? And this has been your home ever since then, since the early 90s?
Yes, I came here out of grad school in ‘93 as a postdoc for a couple of years, and then I was a contractor with SETI until ‘98 and that’s when I got the civil service position.
Why don’t you elaborate a little bit on the work you’ve been doing here at Ames and perhaps some of the discoveries or highlights of your work up until now, what you are pursuing, and like you said, it’s at the core of NASA’s interest in understanding who we are and how we got here, so how your work relates to that and what have been some of the high points along the way?
At ASU my project was to find out where “excess”, or “extra”, amino acids were coming from after researchers extracted and analyzed these meteorites under certain conditions. What would happen is that researchers would extract amino acids from meteorite samples and find a certain abundance of amino acids, let’s call it X. Well, my professor found that if you added acid to that same sample and heated it, the amino acid abundances would then go up by 2X, 3X, or more. Obviously, you were breaking some bonds of unknown precursors and liberating those extra amino acids. So what were those precursors? This phenomenon was of such interest, not just with him but across this whole field because one question was, are you breaking up peptides or some other important precursor to life? For example, biologists know when you take protein (peptides) and hit them with acid you break them down and release their monomer amino acids. So good grief, were there 4.6-billion-year-old peptides or proteins in meteorites? So my job was to find out what compounds these extra amino acids were coming from. So I looked and looked and it turned out that they weren’t peptides, at least none that I could find, but there were a lot of amino acid derivatives, i.e., compounds that had a different small group attached to one end of them. This made a difference for the particular analysis method researchers were using because, and I won’t go into describing the analysis, if the hidden amino acids were bound by a group on one end you couldn’t tell that they were amino acids. So when my professor, and others, were hydrolyzing these samples with acids, they were simply breaking an attachment off unknown amino acid precursors, and then they would see the total amino acid abundances go up. But the amino acid precursors weren’t peptides – they were a class of compounds called amides. However, these amides could’ve also been important on the early Earth. It turns out that when you simply heat these amides, and I won’t go into the names of them, they link together and form these large protein-like chains. Heat will do a lot of things and so they were of interest that way.
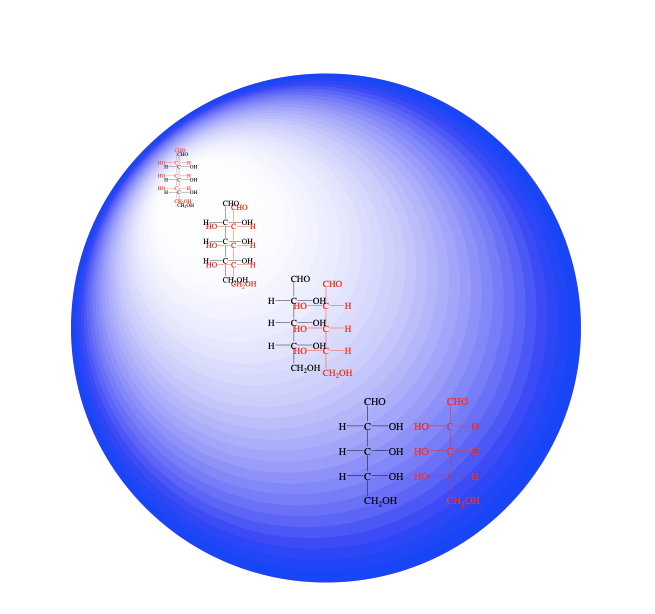
So on to Ames, and I mentioned analyzing meteorites for sugars and sugar derivatives. First, when chemists say sugars, they of course don’t mean common table sugar. The everyday term for “table sugar” refers to a glucose molecule (a six-carbon molecule) connected to another six-carbon molecule, fructose, that’s table sugar. But the actual chemist’s definition of sugar can include compounds containing as few as three carbons. So, I’ll take you back to maybe high school: a true “sugar” contains an aldehyde group (a carbon double bond to oxygen and on that same carbon is one hydrogen) and the other carbons are bonded to an oxygen-hydrogen (-OH) group and one hydrogen or two.
In current biology amino acids make proteins but sugars are in DNA, RNA, and other material. Sugars often form long polymers, and so were there sugars in meteorites? So I started looking, running standards, and I saw the smallest, a three-carbon sugar (dihydroxyacetone), but mostly what was there were the slightly oxidized versions of sugars, or “sugar acids”: Imagine a sugar with one oxygen added to the aldehyde group. So instead of say ribose, the sugar in your RNA, there is actually a close derivative, ribonic acid; instead of glucose, add one oxygen, it is now gluconic acid. Many of these sugar acids are still widely used in biology. Other meteoritic sugar derivatives include sugar alcohols: you hear of them now in diet soda and other foods, for example, erythritol, xylitol, etc. Well, all of these compounds (and other sugar derivatives) were floating around in space before we were here.
Yes, but just one question: help me, from a non-scientist point of view, to understand NASA’s interest in these chemical elements that are found in meteorites, these amino acids that we’re finding, is it related to ultimately how life might have come to be?
Right, right. If you are looking at the first cells on earth or possibly anywhere else, from what we know, you had better have sugars and amino acids or there won’t be any life.
So it’s like water in that sense? We’re looking for exoplanets that have a very narrow temperature range because without water we don’t understand how life as we know it could exist. And it’s the same thing with these sugars? OK, I understand.
From what we know now sugars might be as valuable as water. But, in the future maybe someone will find that instead of ribose there may be some other organic chemical that could have played a similar role and helped to start life somewhere, but you’d have to prove that, and ribose does such a nice job. Every form of life that we know of has that one sugar in its RNA. Since one of NASA’s core goals is understanding life’s origin you want to look for extraterrestrial sugars. Can you imagine taking glucose away from life now? That wouldn’t work. (laughs)
OK, perfect, thank you. And now onto the work that you’re doing now. Does that describe your work, or has it evolved into something else?
My work, for now, comes out of finding the sugar derivatives in meteorites. Using just one compound as an example, and you don’t have to know the structure of ribose, but it’s a five-carbon sugar with each carbon bonded to an oxygen. These, and all, compounds (and all objects) have mirror images. Imagine an object, a molecule, or your hand, in front of a mirror. You’ve probably heard scientists talking about right- and left-hand molecules. Of course both your actual left and right hands, and left and right-hand ribose are real objects. When you go into the lab to make ribose and other molecules in a physically unbiased way from small precursors, both the left and right-hand molecules are going to be synthesized in equal abundance. That is called a natural or non-biological (or abiotic) synthesis: you will get a 50:50 mix of mirror-image ribose
So that, believe it or not, is a huge part of a mystery because right now biological ribose is overwhelmingly found as just one of the two mirror images; the same for the amino acids that are found in proteins. So how do you go from what we thought was a natural 50:50 mix of these mirror-image molecules ~ 4.5 billion years ago to, in most cases, the use of only one in modern biology?
I mean you flip a coin (i.e., do a “natural” synthesis) and you get 50-50. So how did your RNA and proteins take away or start with just one mirror image of the monomers? That’s crazy! And so, such asymmetry was also a mystery even in 1840 with Louis Pasteur. Back then they had crude polarimeters that could rotate the light from mixtures that weren’t perfectly symmetrical (i.e., not 50:50). So they knew that there was something asymmetrical there. They would extract a plant, let’s say, and whatever chemical was there, and there were thousands of them, they would get a rotation of light because, again, biology now makes asymmetry. So they knew there was something asymmetrical – that’s why I say this is a 200-year-old mystery. To this day people have tried to go in a lab and, using abiotic means (e.g., no complicated enzymes allowed), create excesses of one mirror image in the compounds they synthesize: in other words, we’re trying to simulate what could have happened, at some point, billions of years ago that started a path towards the biological use of only one of a pair of possible mirror images. There have been some claims over the decades but nothing has seemed to pan out.
In my lab, we’re doing mainly two things: still analyzing the mirror-image ratios of meteoritic organic compounds and also trying to simulate how this symmetry breaking could have happened in the distant past. For the chemical analysis in both projects, you need special equipment such as asymmetrical chromatography columns, gas chromatographs, etc. The meteoritic sugar-acid analyses included those made of three to six carbons. We saw that the mirror image ratio of the smallest one (glyceric acid) was 50-50, again, the left and right-hand molecules were of equal abundance. So I was actually pretty happy: that meant that the sample was not contaminated by Earth microorganisms, the results really were from space. If you get equal amounts, remember that in a lot of cases nature doesn’t make both mirror images. But after the three-carbon results, things went a little strange. OK, so the smallest one had equal ratios: then we went up to an acid that was made of four carbons. But, instead of 50:50, its right mirror image was ~ 3:1 larger than its left! I said, “Oh, wow, what is this?”. Then we moved up to the acids made of five carbons, including the derivative of ribose (ribonic acid), and instead of 3:1, they were like 6:1 right over left. I tell this joke that after I saw that, I started heading to the retirement office! I said, “Some student has dropped part of their lunch in my samples!” From space, you’ve got these molecules with six times more of the right image compared to the left? How does an unbiased 4.6-billion-year-old process do that, there were no enzymes back then!
However, I stopped halfway on my walk to the retirement office because I noticed that even for the four compounds in the ribonic acid group, one (lyxonic acid) wasn’t found on earth – almost never, I’ll put it that way. And, it too had the same bias as the more common ones in that group. So for the more common one, yeah someone could’ve dropped something in my test tube so it could’ve been contaminated, but not the one that was not found on earth. Then I said, “ OK let’s look at more, let’s move up to the six-carbon compounds, the derivatives of glucose, mannose, etc. So we go from five carbons with their 6:1 excesses up to the six-carbons and that bias was even greater, in fact, there were none of the left mirror images. However, there were only a small amount of the six-carbon sugar acids in the samples so the results aren’t as certain. I want to get a larger sample for a better look at the six carbons. Anyway, you go from the smallest (three-carbon) sugar acid with 50-50 mirror images; move up to the four-carbon at 3:1; five-carbon at 6 to 1; and six-carbon with an even larger excess? It could just be an interesting coincidence that in meteorites the more abundant mirror image of sugar derivatives, and also the more abundant mirror image of certain amino acids, are the same ones that life now uses in each of these same classes of compounds. We published this in 2016.
With Andro Rios, a junior scientist here, we’re doing experiments that try to re-create those differences, those excesses, in organic compounds. Billions of years ago, what forces could’ve caused excesses when previously everyone thought in “natural” settings you’d get an equal amount of mirror images? And that’s what we’re funded for, we’re doing a variation of the “formose reaction” with forces such as radiation.
Well that’s good and you were joking that it almost led you to retire, and I was thinking the opposite: it gives your job security, that’s for sure!
We’ll see. Oh, and that is what scientists have been trying to do, like I said, for the last couple of hundred years: make mirror-image excesses from natural and simple molecules. I think we’ve done it, it’s just a matter of now doing the same experiments fifty times so people are happy with your statistics.
We can certainly feel your enthusiasm and passion for the work you are doing so here is a curveball question: if you weren’t a NASA research scientist what would your dream job be?
You know, I would go back and take a lot of math and physics and then just do some combination of what I’m doing now with a heck of a lot more math.
That’s interesting. It’s not too far removed from what you’re doing now and you’re certainly comfortable in your avocation.
What advice would you give to a student, for example, who looks at your career and would like to have that kind of professional life?
Well my pretty mundane advice is, whether you are at NASA or not, if you’re at a university and want to do this type of thing, just study chemistry and physics and if they know they want to do this kind of stuff then they probably already like those types of subjects, so you don’t have to tell him to be enthusiastic and all that. I would say most senior chemistry and physics majors like what they’re doing.
OK, I’ll reach a little wider then. You’re obviously focused and busy with your work but what do you do for fun?
Well, as far as being focused on my work, I’m focused on a lot of meetings right now that have nothing to do with my work! (laughs). But for fun? I exercise and go hiking, things like that. I actually try to read when I get time. For Physics, you know chemistry majors only have one or two courses, so actually I grab my physics book whenever I can and read a lot of that stuff because it’s going to help me now. I wouldn’t call it fun but it is sort of fun to go back and say “Oh, that’s what that meant! OK.” You never really needed to know a lot of what you read as an undergrad but now it will at least help you in the background. So, exercising as much as I can, hiking, and reading a little science.
What book might we find on your nightstand? Are you interested in a particular genre? I don’t want to prejudice your answer but quite a few of our researchers read a lot of science fiction, for example.
I probably would read more areas but I’m always trying to catch up on background science that applies to the particular lab work I’m doing at that time. Over the years I always say that I’m going to read more of my history books, etc., but I’ve had to be in the lab constantly because lab researchers here, e.g., chemists, biologists, are not like university professors. We don’t have a gang of grad students in there working and we can’t hire permanent staff. Even with his other duties my professor could really get up to date faster because his grad students and postdoc were in there working. I don’t know if he knew where the lab was! (laughs). Here, you’re trying to keep interns in the lab for beyond a semester. So as a lab person, as a hands-on lab person, you don’t get as much time to do other things. You better be in there on most weekends doing some of your more laborious stuff, or the research isn’t going to move very quickly.
Has the Covid shut down affected your time situation so that you’re saving a lot of time that you’re not commuting or do you live close enough that that’s not really an issue for you? Has it given you more time to do things that you like to do since are you working remotely?
I still have interns and so when the shut down first started (and now) we would meet outside and by computer: we could go through this ton of data we have, and we’re still going through it. Now, outside is fine, especially in the summer. So a lot of time that we would’ve had in the lab was taken up by data analysis. Also, it seems like other people want you to be on committees and panels also. I just got through with an all-day NASA panel.
Has your work so far involved very much fieldwork or is it mostly analysis of samples that you get from various sources?
Right, the latter. In my case, it’s the lab work, analysis, and simulation experiments. The field workers go off to the desert and all that but I’m not one of those. My meteorites, I request them from museums, etc., people that do fieldwork to go out and collect them. One of the panel members from yesterday goes to the Arctic all the time and looks on the ice sheets for meteorites. But I just request them. I’m a lab person.
And you don’t like the cold!
Yeah, and it takes, I imagine, a whole bunch of training. I’m not sure what they go through to be able to do that, but they love it.
What accomplishment are you most proud of that is not science-related?
Oh! Not science-related. Hmmm. Boy, that’s. . . Well, I have a big upright bass in the living room and I’m proud that every day I walk past it and say, “I’m going to get on that one day and continue practicing!” (laughs)
We also kind of ask two things: do you have a favorite image of space or science or something representing your work that’s meaningful to you, that we might see on the wall of your lab if we went in there? And along with that: who or what inspires you?
Well first an image: I have several posters and I like the science. Some of the Hubble images are literally out of sight. But that’s the kind of thing I have on my walls.
Who or what inspires you, or has inspired you along the way?
I don’t know if there’s a particular who or what but I like looking at, well I don’t have cable anymore, but I enjoyed looking at things like the Discovery Channel and astronomers and astrobiologists. It was fun years ago to watch Carl Sagan. But for particular inspiration? The compounds, the phenomena we are seeing, the mirror images, I can bury my head in that constantly and that would be enough. To know that you’re looking at these things that are 4.6 billion years old, and that makes no sense, so that’s motivating.
I’m curious, are there always sugars in meteorites or is it not that common?
There are more organic compounds in what are called carbonaceous meteorites compared to others. They have more carbon than the more heated meteorites. We looked at several of the carbonaceous meteorites and most have some amount of sugar derivatives. If I were to analyze a lot more meteorites I would probably find more compounds. However, we analyzed a good little selection of the carbonaceous and that’s what we published in 2016.
That means there’s a lot of it right?
Well I mean you go to the grocery store and you buy glycerin. Well, that’s glycerol, another sugar derivative. A minute ago I talked about the smallest three-carbon sugar, glyceraldehyde, and glycerol is related to three-carbon compounds. It is the most abundant of the meteoritic sugar derivatives, more abundant than the amino acids. That also could have been important on the early Earth, it turns out that it is a nice high-temperature solvent for doing reactions.
Well, George, we really appreciate spending time with you here. Thank you so much.
Thank you.
Interview conducted by Fred and Sara on 4/14/21 virtually.
Learn more about George Cooper’s work here.