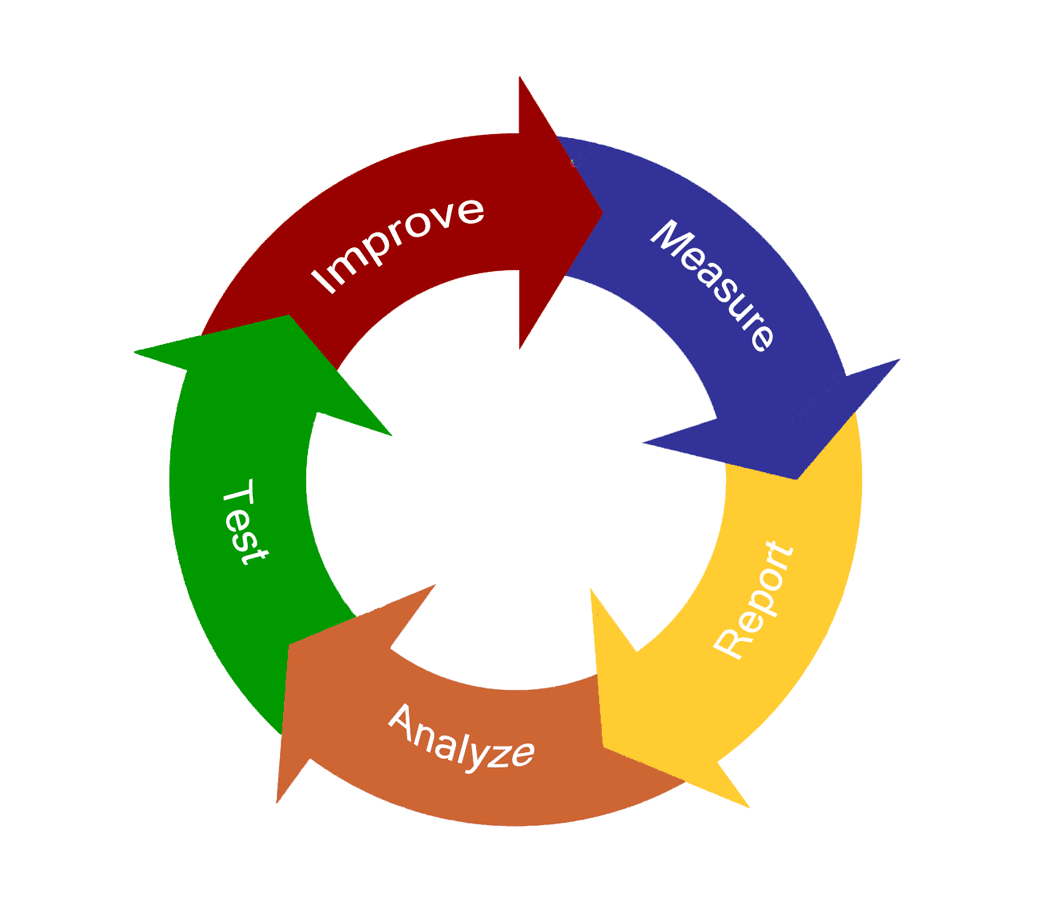
Continual improvement is the mainstay of our Quality Management System as we work to improve our product quality, employee communication, work environment, resources, and customer satisfaction. Employees at all levels of our organization participate. Our senior management provides the leadership, support, and resources to make continual improvement a priority. Middle management and process owners focus efforts and resources and review progress, and employees identify opportunities and recommend improvements.
Information about improvement opportunities are gleaned by management representatives for presentation to top management. Our management reviews and evaluates the data to determine and, if necessary, implement changes to improve products, processes, and resources. This information comes from a variety of sources.
Review and Operations Meetings
Opportunities for improvement surface during process operations meetings when quality measurement results are reviewed and compared to quality objectives.
Data Analysis
Data analysis can provide significant information on operational performance and improvement opportunities. Management must review and make decisions and take actions on the results provided by such data.
Audits
Results of product, process and Quality Management System audits provide many opportunities to improve Quality Management System effectiveness and efficiency. Opportunities may relate to communications, information systems, processes, controls, use of resources, technology, etc.
Corrective Action
Corrective action is action taken to eliminate the cause(s) of a detected nonconformity to prevent recurrence. Most businesses experience problems on a day-to-day basis that may relate to products, Quality Management System processes, resources, suppliers and outsourced work, product shipped to customers, customer complaints, etc.
Preventive Action
Preventive action is action taken to eliminate the cause of a potential nonconformity or other undesirable situation to prevent occurrence.
Management Reviews
The purpose of conducting management reviews of the QMS is to gauge the health of the Quality Management System suitability, adequacy, and effectiveness.