A crew member “drops” his or her sandwich aboard the International Space Station (ISS), and it hits a surface. Quick! Grab it within five seconds or it is spoiled?
If this rule really did apply, and the sandwich was picked up few seconds too late, not much bacteria would be found, according to the latest published results of the Lab-on-a-Chip Application Development – Portable Test System (LOCAD-PTS).
The paper, titled “Rapid Culture-Independent Microbial Analysis Aboard the International Space Station (ISS) Stage Two: Quantifying Three Microbial Biomarkers,” provided molecular data on the distribution of microbial molecules – parts of microbes that are single-cell organisms – in every livable space of the station.
“This means that the LOCAD-PTS analyzed samples collected from surfaces in every habitable module that was part of the ISS from 2007 to 2009,” said Heather Morris, LOCAD-PTS scientist at NASA’s Marshall Space Flight Center in Huntsville, Ala. “The molecules that it can detect are cell wall molecules of bacteria and fungi.”
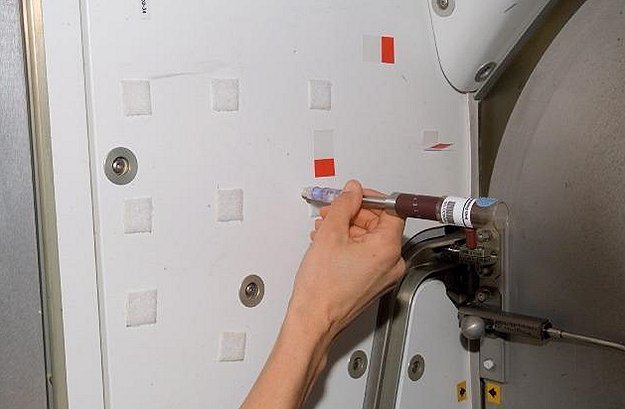
The handheld LOCAD-PTS device rapidly detects biological and chemical substances on surfaces aboard the station. Astronauts swab surfaces within the cabin, mix swabbed material with a liquid before adding it to the LOCAD-PTS and obtain results within 15 minutes on a display screen. The lightweight system has three different types of cartridges for detecting endotoxin, a marker of gram-negative bacteria; glucan, a type of fungi; and lipoteichoic acid, a marker of gram-positive bacteria. The category of gram-positive bacteria includes multiple pathogens, specifically the well-known Streptococcus and Staphylococcus, making the identification of these bacteria important for crew health.
The study showed a rapid indication of biological cleanliness to help the crew monitor microorganisms in the space station environment. Likewise, this technology can provide a quick screen for bacterial/fungal contamination in a hospital or other clinical setting, particularly after cleaning, to assess the sanitization of the surface.
“The major benefits of the LOCAD-PTS are the low, relative cost and minimal crew time to operate with rapid results,” said Norm Wainwright, Ph.D., the principal investigator for the system from Charles River Laboratories in Charleston, S.C. “It can be used right at the place where contamination may have occurred, only requiring a small sample, and performs the analysis in a contained environment with no growth of organisms required.”
In the paper, investigators reported that most surfaces used by the station crew are relatively free of microbial molecules. However, the number of microbial molecules were elevated at sites frequently contacted by crew members, including the workout bike in the U.S. Laboratory module; the Japanese Experiment Module (JEM) airlock handle; and foot rests, drawers and the waste and hygiene compartments in the Zvezda and Tranquility modules.
The first molecular test of surfaces in the newly docked JEM were performed using the cartridges designed to detect gram-positive bacteria, including the microorganisms that can cause staph infections and strep throat. The results revealed relatively clean surfaces.
“By and large, we found that the station is a clean environment as the crew does have a stringent cleaning protocol,” said Lisa Monaco, Ph.D., scientist for the LOCAD-PTS at Marshall. “Our in-orbit testing revealed some areas of hardware/software/testing modifications that need to occur, and we’ve begun working on all of them, but overall we’re very satisfied with the results.”
Simple panels on walls generally returned low readings of microbial molecules, but sometimes sleep stations would give the astronauts both high and low results.
“We wondered if that was due to the roughness of the surfaces and our swab,” said Wainwright. “Sometimes it would collect more, perhaps because of the way and the angle the swab was used…[P]anels are flat, smooth surfaces, so if a molecule or a cell is on them, it’s going to be a little easier to pick up. But if it’s on a rough, corrugated surface, they might be buried down into something. So we’d see variations mostly with fabrics.”
The crew compared results from the LOCAD-PTS with those obtained by the current standard protocol. This method requires pads of gelatinous, agar-based media called contact slides to be held flat to a surface for one to two seconds to capture any microbes. After a sample is taken, the astronaut incubates them for five days and then photographs the slides to capture any colony growth.
“While the data from LOCAD-PTS did not directly correlate to the colonies that we observed growing on the contact slides that had sampled sites close to the ones that we sampled, both tests did provide similar results some of the time,” said Morris. “And there are explanations for the differences. Microbial density varying across a surface or differences in sampling – contact slide versus swab tool – could account for some of the discrepancies observed.”
By having cartridges that could detect all three classes of microorganisms, this study greatly expands the usefulness of the LOCAD-PTS, which had previously only been able to detect gram-negative and fungal contamination.
Currently, the technology is being used to assess fluids used in pharmaceutical processing. It also will provide environmental testing capabilities that may serve homeland security. Additionally, future iterations of the technology could provide rapid medical diagnostics in clinical applications.