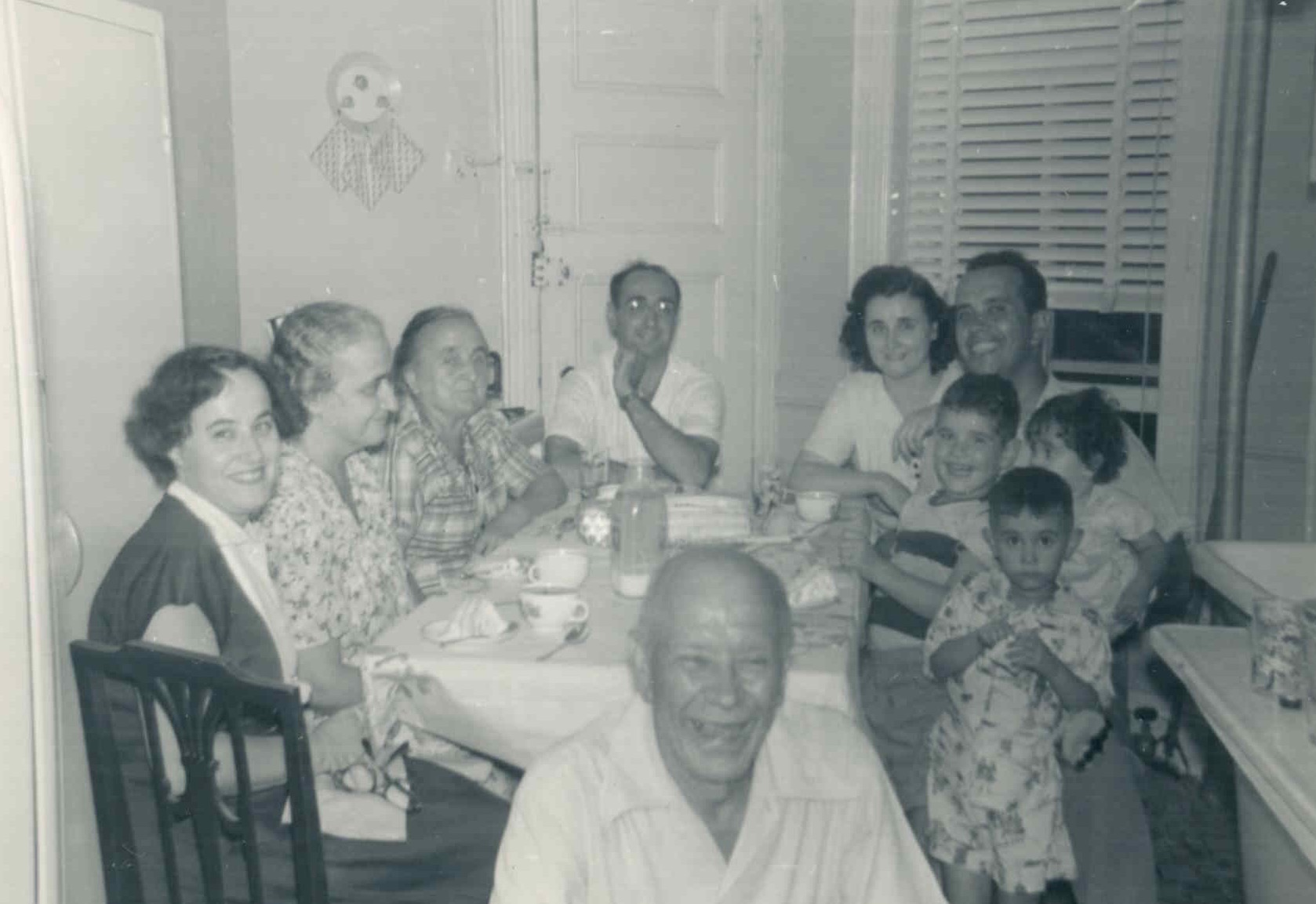

Good morning Lou. It’s so nice of you to carve out a few minutes with us for this interview. I looked at your bio and history and the farthest back I could find was that you went to a school in New Jersey, but I don’t know where you were born and grew up so why don’t we start with that, with your childhood, where you’re from, your memories from back then, and especially if there was anything in those very early years that might have a pointed you toward the career that you have pursued.
OK, well, my story is rather unusual, with significant and surprising twists and turns. It starts with my grandparents. All four were Italian immigrants who came to the U.S. through Ellis Island in the early 1900s. I was born in the Greenwich Village section of New York City, on Manhattan Island. It was a vibrant Italian neighborhood then, not the chic place it is today. And although still known as Little Italy, it’s a very far cry from its former self. But as a little boy that was my neighborhood, with relatives from different parts of Italy not too far away, Italian mostly spoken on the streets, and Italian festivities punctuating the year. The first picture was taken on my fifth birthday. I was born in 1946 shortly after World War II and went to St Anthony’s grammar school, an Italian Catholic school on Houston Street. New York City was a very safe place back then. I would be out on the streets with my friends all day Saturday, like one of the kids you see in those old pictures running around in front of gushing fire hydrants on a hot summer day. Or playing stickball (baseball) in the street since there wasn’t much traffic; avoiding the occasional car, or even a horse-drawn grocery wagon. I have wonderful memories from those days. We moved 15 minutes away to New Milford in New Jersey when I was in the fourth grade (1954), to what seemed a world away. Only a few of my relatives took that trip all the way across the Hudson River to visit us in “Joisey”. It was only 15 minutes away but some of them never crossed the river because it seemed so far away! Now I’m out here in what we used to call the Far West, oh my Lord, were those really different times! They were great years, right after the war, and many of the men I knew were World War II veterans.
Did you have brothers and sisters?
I was the oldest. I had one sister, born in 1950, and a brother born in 1951. They were rather young when we moved to Jersey and it was a very idyllic life. Again, we’re talking about 1954, in an older suburb in Bergen County. There I went to St. Joseph’s grammar school in Oradell, where Wally Schirra, one of the seven original NASA astronauts, grew up. The house was huge compared to the apartment in New York, with lots of wooded areas and waterways nearby. I loved fishing, I loved being outdoors exploring these places on my bike, and I loved being a Boy Scout and working on Merit Badges. That curiosity has lasted until today. I don’t know how general this was, but I didn’t have science classes until the 7th grade. Sputnik went up in 1957 and that changed everything. We had science classes with shiny new science books the next year, my seventh grade. I just loved that book. We had English and math and all that other stuff, but the science book fascinated me. It had skeletons with labeled bones and all sorts of things. That book, as well as the science fiction books in the town library, had a large impact on my life.
I went on to Don Bosco Prep, an All-Boys high school in Ramsey NJ. I imagine my high school years were similar to most with two major highlights: I played football and loved being on the team, and I met Mary, my future wife, in spite of the fact that she attended an All-Girls high school miles away. Meeting Mary was the most important thing that happened to me.
My goodness! Give us the short version of how you met.
Oh! (laughs) well, it’s a delightful story. Don Bosco is a commuter high school with students bused in from all over Northeastern Jersey. The fellow behind me in one of my classes invited me to come to their Catholic Youth Organization activities at his church. It was only some 12 miles away and I accepted. The first night there was this girl who caught my eye, and apparently, the feeling was mutual. We were both shy and I don’t think we said much to each other during the first few evenings. My matchmaker friend would tell me “That girl that you mentioned, she’s very interested in you”. And coincidentally, he was telling her the same thing! He then set up a blind date for me with a friend and Mary was my blind date’s best friend, so we all went on this date together and that’s how it started.
And the rest is history!
Yes, the rest is history!
You got set up, that’s what happened!
Yup, in the most fortunate of ways. And a very good antidote to a lowlight during my senior year when one of the teachers I had asked to write a letter for my college applications told me it wouldn’t be a flattering letter because he didn’t think I was “college material”. I suppose he thought me a ‘jock’. I did love football and lived for our Sunday games, but I also realized school work suffered during football season. Science was hardly on my radar during my high school years.
In 1964, in spite of not being college material, I went on to Saint Peter’s College in Jersey City, an outstanding small, all-male, Jesuit school. My freshman year started with a terrible tragedy. While visiting the College with my mother on Parent Introduction Day, my father died of a heart attack. That was an awful time and it took me quite a while to recover. The Jesuits at the college were very supportive, assuring my mother not to worry about college costs as long as I kept a good grade point average. My mother, a homemaker since her marriage, now had to support the family. Had it not been for St. Peter’s generosity, who knows how my life would have turned out. As you might imagine, I grew up very quickly after that.
So, you were in college there in New Jersey and then you somehow wound up working on a Ph.D. out at Berkeley. How did that come about?
As I just mentioned, I grew up very quickly and blossomed at St. Peter’s. Tucked into a few city blocks in Jersey City and not noted for its campus, St. Peter’s ranked among the best small schools in the country. It was a great liberal arts school with very high standards. I discovered I enjoyed learning very much and blossomed academically. I commuted for the first two years, rented an upstairs room in a local house for $10 a month for the third, and lived with four roommates in a basement apartment the fourth. I chose chemistry as my major for no particular reason other than my interest in science and, it being a Jesuit school, I had to minor in philosophy and theology.
While I thoroughly enjoyed nearly all of my classes, once I started to understand the basics, I just fell in love with chemistry. It was here where I met two of the men who greatly influenced my life: Fr. Hilsdorf, the Chair of the Chemistry Department and my freshman chemistry professor, and Dr. Pegolotti, my organic chemistry professor. What I learned from them, in and out of class, has figured prominently throughout my life and career. Fr. Hilsdorf was a very experienced Jesuit and seasoned chemist. Dr. Pegolotti was a young man who had earned his Ph.D. at UCLA a decade earlier. Both of them encouraged and prepared us to go on to graduate school. Many of us were the first in our families to attend college. Left to our own, we would not even have considered grad school a possibility. As a side note, I am still in contact with Dr. Pegolotti. He now lives in North Carolina. Thanks to the education, reputation, and support from St. Peter’s, incredibly, I was accepted into the Chemistry Department at UC Berkeley. I still remember the day the envelope came with the acceptance letter from far away Berkeley, California! The place where they created elements! This was huge to this Jersey boy. In those days transcontinental trips from Coast to Coast were rare, perhaps once in a lifetime for most people. People still ask me, “How did you get into Berkeley?” My answer, “Thanks to Dr. Pegolotti and St. Peter’s College, I didn’t know better and applied”.
So, Mary came out to Berkeley with you when you went to school here?
Yeah! We married the week before my graduation from St. Peter’s. Mary had just finished three years of physical therapy training at Columbia in New York. We spent the summer in Jersey and, in September 1968, we flew from Newark to San Francisco. It was my first plane trip, and boy, was the Bay Area very different from Jersey. The big initial shock for us was the difficulty in finding a place to live in Berkeley. The housing office was bedlam and we ended up living several miles away in Oakland because that’s all we could afford that first year. I was a teaching assistant and Mary was expecting our first child, Monica. Eventually, we had four children: Monica who was born in Berkeley; Patrick in Corvallis, Oregon; and Kees and Anthony, who were born in Leiden, The Netherlands. I’m getting ahead of myself.
OK, so you got to Berkeley, and then at some point, you chose a major that led to your science degrees so tell us about how you decided to pursue that major.
After the initial shocks, with the Berkeley riots in the background, we settled into a good, grad student life in California and with time I fell in love with the place. But the first couple of years adjusting to life in the Chem Dept at UC Berkeley were difficult. There were times that first year I didn’t think I would make it. Being an honors student at St. Peter’s in Jersey City and getting through thermodynamics at UC Berkeley was a whole different animal. But I made it, and, in the end, I worked under Professor George Pimentel on several projects in physical chemistry. Physical chemistry is not what most people think of as chemistry. It generally doesn’t involve Erlenmeyer flask’s, distillations, and mixing liquids as characterizes organic chemistry. Rather, it is a blend of chemistry and physics. It’s called molecular physics in many parts of the world. Coming to Berkeley I thought I would do graduate work in organic because of Dr. Pegolotti’s influence. But wandering around the various sections of the department, I was surprised by the large vacuum chambers, oscilloscopes, electronic instruments, huge magnets, things with levers and valves and pumps, and remember thinking, “I don’t know what these have to do with chemistry, but I want to learn how to use this stuff.” It’s strange, I didn’t have a clue of how it all fits together, but I had a strong natural affinity to work with that equipment rather than sitting at a lab bench, mixing things and taking notes. Which I did like.

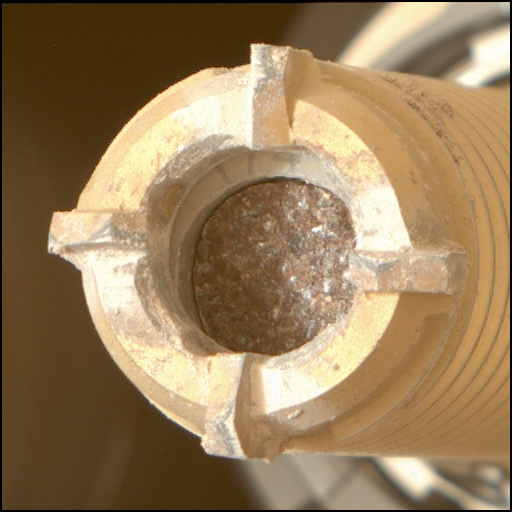
After a two-year false start with another professor, I joined Professor George Pimentel’s research group. George was a wonderful man, who was extraordinarily gifted and energetic. His wisdom, optimism, and guidance in life, and research have remained central throughout my life. My research focused on UV-driven chemistry at -420o F, some 22° K above absolute zero. At these temperatures, just about every gas is frozen solid. Since this was before liquid helium dewars and closed-cycle helium refrigerators were available, we used liquid hydrogen as the coolant, the fuel NASA uses for its rockets. I doubt that would be allowed in many labs today. Let’s say we wanted to study how a gas like oxygen or carbon dioxide behaved when it was in the solid-state and exposed to UV. We first froze the gas onto a surface cooled to 22 K and measured its infrared spectrum. We then irradiated the solid-gas with UV and took another infrared spectrum. Since the infrared spectrum measures chemical composition and structure, the before-and-after changes show what chemicals were formed and what were lost. George was among the pioneers who developed infrared spectroscopy and these low-temperature studies into a chemical tool; I was taught by a master. To carry out my particular project, I had to build a room-sized apparatus to synchronize gas pulses flowing to the cold window with high intensity, 20,000 volt, UV lightning flashes; all less than an inch away from two liters of liquid hydrogen. Dubbed “The Marvelous Machine” by George, it’s shown in the second picture. I’m the curly haired guy adjusting the gas flow standing in front of the asbestos, ‘fire-proof’ curtains. I learned so many things building this: infrared spectroscopy, glass blowing, high vacuum techniques, handling liquid hydrogen, electronics, machining, and a lot about high-energy radiation.
Personally, those Berkeley years were good years. Mary and I traveled around the state, enjoying the incredible natural wonders from the Sierra to the northern redwoods on a grad school budget, which meant lots of camping. I became enthralled by the High Sierra and had great times backpacking and climbing to get away from the lab. It took five years to complete my doctoral work.
In 1974 we moved to Corvallis, Oregon where I began postdoctoral work with Professor Joseph Nibler at Oregon State University. Joe, a superb experimentalist and IR spectroscopist, broadened my horizons even further. He had a very modern lab. Instead of using liquid hydrogen as a coolant, he was using one of the first closed-cycle helium refrigerators on the market (serial number 4) to cool the sample windows and, in addition to infrared spectroscopy to probe the samples, laser radiation to measure Raman spectra and induce fluorescence. Again, I found myself designing and building a new instrument, this time to excite compounds in the frozen sample with pulses of laser light and to track how they relax using pulses from another laser.
Patrick was born during our first year in Corvallis, a very small town then, with a population varying from roughly 17,000 to 33,000 depending on whether or not the University was in session. Living in a small town was a very novel experience for Mary and me. Again, we enjoyed traveling around the state, exploring the rugged coast and high desert. However, now with two small children, so not as carefree as before. Our student days were gone as were our student discounts. At the time, students were entitled to many discounts from movies at half price, to student rental rates. Discounts were common and we could survive on $400-500 a month in Berkeley. That was no longer true and it was time to look for a real job.
And once again, here’s when another strange, surprising twist of fate made all the difference. The country was entering a recession because of the 1973 oil crisis and there weren’t many jobs to be had, let alone for a specialist in chemistry at -420o F. As reality sunk in, Mary and I even started thinking about opening a business, perhaps a little Italian restaurant or pizza shop. Coming from New York, Berkeley, and Corvallis pizzas just didn’t measure up. It was then that I got a call from George (Pimentel) that, yet again, set our lives on an entirely different course. After the usual courtesies, he asked if I was still looking for a job. I said something like, ‘George, I wouldn’t be here if I wasn’t looking for a job.’ He replied, “Well, I’ve heard about a job that has your name written all over it. I’ve been contacted by Mayo Greenberg, a professor who is looking for someone with just your talents and interests. He’s a theoretical astrophysicist who studies extremely cold dust in deep space. He wants someone to develop a laboratory to simulate the ices that form on that dust and study their chemistry.” George knew I was fascinated by the exciting space exploration of the ’60s. He went on to explain Mayo theorized that since most of the gas in deep space should condense (freeze) onto the small, extremely cold dust particles that are present, these ices should play very important, but unknown, roles in astrochemistry. Naturally, I became very excited until he asked, “How’s your Dutch?” I was speechless. He filled the silence with “Lou, Dutch is the language they speak in Holland.” “I know that I replied, what’s that got to do with anything?” He said, “Well, the job’s in Holland!” So, imagine: we’ve got two kids, it was not that long after World War II, and Europe was still in recovery. We’d heard all kinds of stories of what it was like over there, so the thought of going to Europe, let alone for a job, was even more rare than going to California from New York. Most of my grad school colleagues advised against it. But, after many (handwritten) letters back and forth with lots of questions thoroughly answered, Mary and I were debating it seriously. I remember the day we were talking about it yet again, when she said “Lou, maybe we should give this a try. Perhaps it will be OK and all work out. If not, we can come back and we’ll be no worse off than we are now.” With this perspective, we went to The Netherlands in 1976. It was the best thing we’ve ever done. It changed our lives profoundly.
The job was at Leiden University Observatory, one of the great astronomy departments in the world. The Dutch are very disciplined, polite, and social, with strictly scheduled coffee, tea, and lunch breaks daily. So, there I was, sharing these breaks every day with enthusiastic astrophysicists at every academic level, including some of the great astronomers of the 20th century, with absolutely no preparation in astronomy or astrophysics. Given my solid grounding in chemistry, I appreciated how fortunate I was to be in this world-renowned observatory, yet not intimidated. My knowledge of astrophysics doubled every day, it was wonderful.
A little background. Prior to the 1960s, space was thought to be chemically barren, with the harsh UV radiation field and extremely low temperatures thought to preclude even the simplest reactions. This spell was broken in the 1960s with the discoveries of the hydroxyl radical (OH) and simple molecules ammonia (NH3), and formaldehyde (H2CO) in deep space. Although they were found floating freely in dark areas of the sky known to contain dust particles only a few degrees above absolute zero, astrochemical models didn’t consider their freezing on the dust. Mayo Greenberg had this vision that ice chemistry was an important part of astrochemistry, perhaps even producing pre-biotic molecules! A very bold idea he promoted vigorously in the 1970s despite the fact that astrochemistry was just in its infancy and the largest molecules found contained only a handful of atoms. As a theoretical astrophysicist, he knew it was critical to simulate these low-temperature ices in the laboratory to provide data to test theories and develop models. And there were some crazy ideas floating around at the time. To this end, Mayo came to Leiden in the early ’70s to start the Laboratory Astrophysics (Laboratorium voor Astrofysica) group and I was fortunate to be part of that group. Together with Fred Baas and Ewie de Kuijper, and working with outstanding support staff and graduate students, we built the first laboratory exclusively dedicated to the study of interstellar ices.
The Dutch taught me many invaluable things about designing, building, supporting, and running a productive research laboratory during those years, both on a human and a scientific level.
It was a very exciting time, interstellar molecules were being discovered at a rapid pace, astronomers were now measuring partial infrared (IR) spectra from objects in deep space, NASA had recently started flying the Kuiper Airborne Observatory (KAO) to measure the rest of the IR spectra obscured by atmospheric water and we were using IR spectroscopy to track the chemistry in low-temperature cosmic ice analogs. Not only could we use our rapidly growing collection of IR spectra to unravel ice chemistry in the lab, but by 1980 we were using them to interpret the spectra measured with these telescopes. Comparing our lab spectra with some of the first telescopic spectra of several protostars embedded in dust clouds strongly suggested that mixed molecular ices containing water, ammonia, carbon monoxide and organics like methanol were common in regions where stars and planets form. This picture was borne out over the decade as the number of telescopes and laboratories dedicated to IR studies increased. The critical importance of ice chemistry to astrochemistry is widely recognized now, but I can tell you we were on the margin during the early years. It took nearly two decades before ice processes were introduced into the most widely used astrochemical models.

These were very special, life-changing years personally as well. The third picture shows us about 20 minutes from our home. Our family grew with two more children, Kees and Anthony, I had ‘tenure’, and life was good. As time passed, while Mary and I seriously considered staying in Leiden, a number of personal and professional considerations motivated us to return to the States. But we have good friends there and we’ve gone back on sabbaticals over the years, it was just a great thing for so many reasons.
OK, so you had this wonderful seven-year experience there in Leiden, and now finish it out with the connection that brought you to Ames.
OK! While I had not yet started the search, it was thanks to meeting Ed Erickson, Mike Werner and, I think, Fred Witteborn in 1980 at the IAU Symposium on IR Astronomy that eventually brought us to Ames. That’s where I gave a talk showing the remarkable match between the IR spectra of protostars with our laboratory ice spectrum. Ed, Mike, and Fred were among the pioneers flying on the Kuiper Airborne Observatory at Ames who were measuring these interstellar spectra. In one of our conversations, Mike Werner said “If you’re ever looking for a job you should apply to NASA Ames”. Xander Tielens, one of our first graduate students in Leiden and now an NRC Fellow working with Dave Hollenbach at Ames, also urged me to come to Ames.
A year or so later I started looking for a job in the States, and again it was very difficult and discouraging, to say the least. I still have a folder of over 80 rejection letters from that search. Although I had tenure at Leiden, the one academic offer I did receive required me to go through the tenure process for peanuts and I was not about to put my family through that. By then I had also applied to Ames. I was told they couldn’t hire me as a civil servant, but perhaps as a senior NRC which, if all went well, might then be converted to a civil service position. The proposal would be to build a laboratory to support the Kuiper Airborne Observatory. Although the NRC rejected my first application, Mike encouraged me to reapply and that succeeded. Ames was the natural place to be. As I walked through the Space Science building for the first time in 1983, I was surprised to see the names of so many authors of the papers I had read in Leiden. Now I could collaborate directly with the observers and theoreticians driving IR astronomy.
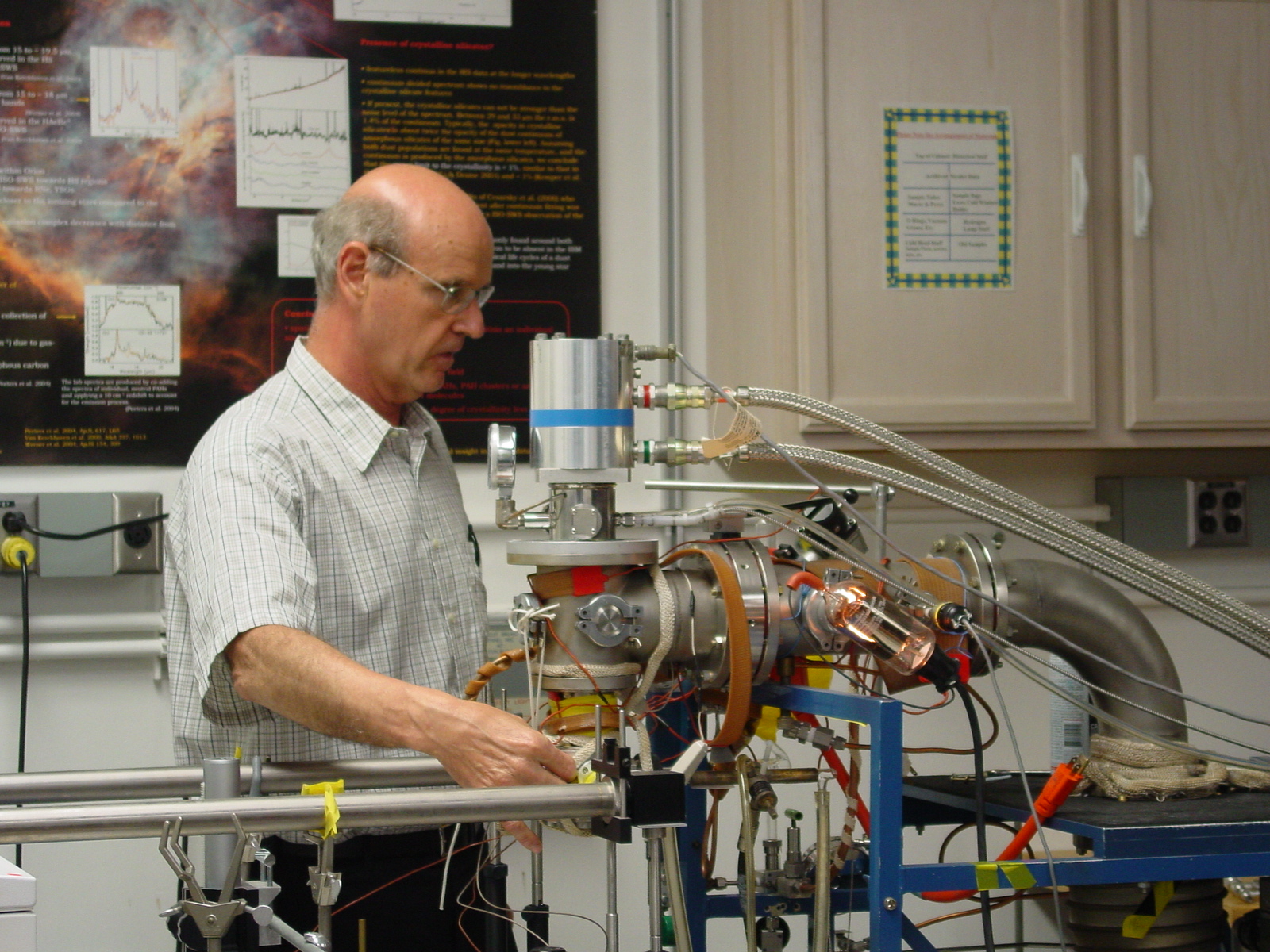
The environment in the Space Science Division at Ames was very open, encouraging, and optimistic. I was immediately accepted into the KAO community and was learning from people like Fred Witteborn and Jesse Bregman who made the instruments and did the observations. With strong management encouragement, Scott Sandford, Bob Walker, and I built the Ames Astrochemistry Laboratory in the ’80s. The fourth picture shows me adjusting one of the cryocoolers. Those first years were difficult, learning how to deal with bureaucracy at nearly every turn and breaking into the funding cycle. With continued management support, and having been blessed with an extraordinary number of gifted NRC and NASA Postdoctoral Research Fellows starting in the late ’80s, its footprint quadrupled over the years. The laboratory cosmic ice simulations expanded to include comets, icy moons and planets. The interstellar polycyclic aromatic hydrocarbon (PAH) hypothesis was born, developed, tested and refined with observations, clever laboratory simulations, and computational studies. It was the interdisciplinary environment in the Space Science and Computational Chemistry Divisions that fostered this truly extraordinary body of breakthrough work. Truly interdisciplinary, close working research groups are very rare.
So, Ames is obviously a government facility and one of the questions we always ask is, your work legacy is well-known and spectacular, but in terms of simply justifying it to the taxpayers, what is the value of the work you are doing, looking at these tiny ice and dust grains at low temperatures way out in interstellar space? How does that benefit humanity?
How does that benefit humanity? It addresses the big questions! It feeds the imagination and gives hope for the future. Let me think. Well, the simple answer is that it supports the space program. NASA decided that the Kuiper Airborne Observatory was a great idea, as is the science of SOFIA and the soon to be launched, eight billion-dollar, James Webb Space Telescope. Those observatories were built to explore the IR radiation from space. There is no point to build those observatories without laboratory research to analyze the data and discoveries they return and help design follow-up missions. It’s a step-by-step process, the same as sending people to explore the Moon and eventually Mars. In our case, the unique collection of PAH IR spectra we generated to test and develop the astronomical PAH model is also important to medical and nano-material research. Many requests for these spectra come from hospitals, environmental groups and chemists. I would answer the American taxpayer that any great civilization should leave something in its legacy for the future.
In 1975, the composition of interstellar dust and ice were guessed at and only a handful of people in ’the business’ thought they were worth studying. The idea of interstellar PAHs would have been laughable. Today ices are seen as playing key roles in the astrochemistry of star and planet forming regions and in the chemistry on budding planets; PAHs play central roles in many astrophysical processes themselves. These discoveries proved that the chemistry of space is far more complicated than anyone could have imagined years ago and contributed to the birth of astrobiology. The Astrochemistry Laboratory was an important part of NASA’s Astrobiology program addressing questions such as “When and how did astrochemistry become astrobiology?”
What is a typical day like for you? Well actually you’re retired now, so a typical day for you is on a hammock with a lemonade, (laughs) but you still go to Ames once a week or so. So, what did you enjoy most and least about your work at Ames?
That’s an easy one. What I least like is, of course, what everybody does: the endless, mindless bureaucracy! Working part-time as a contractor is not bureaucracy-free, but it is much less onerous. What I love, and I think it’s what everybody loves, are the amazing things we are able to work on, tackling new problems, and trying to learn the secrets of our marvelous creation. There aren’t many places where you could do something like that. What a gift!
What would your advice be to a young person starting out who would like to have the kind of career that you have had and are having?
Given my life’s trajectory, I would say that you can’t really plan. I know that runs counter to conventional wisdom but with me, it was just one surprising fork in the road after another with the paths I thought I’d be facing not an option. If I had a firm vision of where I wanted to be in 10 or 20 years, I would’ve constantly been surprised, frustrated, irritated and upset! So, how to put this into words? Just try to be open to surprises and be true to yourself. Put things in the context of the bigger picture.
I hear exactly what you’re trying to say because my career was mostly what happened and what was available at the time. I made one or two-directional choices but mostly I did what I was asked to do and tried to do the best job that I could with the opportunity that was at hand.
Yes, that’s a good way to phrase it. During the oil crisis, a close friend gave me this advice, ‘just keep doing the best you can and, when possibilities open up, make the best choice you can with what you have. It will work out’. Similarly, after I had a disaster in the lab and walked into George’s (Pimentel) office crestfallen, he told me, “Lou, you’re not going to win them all. Don’t let the ones you lose keep you down for too long. Go home, recover, then get back to work.”
OK, here’s a slow pitch: what do you do for fun?
The answer to this question changes with the years. My health is taking over. I still love the outdoors, camping, back-country skiing and such, but the ticker is interfering with that. What hasn’t changed over all the years, is doing things with family-from camping with the grandkids to crossing the Atlantic with Mary on the Queen Mary. No more climbing to mountain tops.
Who inspires you? Or rather, who or what inspires you, or has inspired you along the way?
My mother and father who taught me how to live. Jim Pegolotti from St. Peter’s and George Pimentel from UC Berkeley have been very inspirational. Both broadened my perspective and extended my horizons in ways I would never have imagined and inspired confidence in my abilities that never would have blossomed otherwise. I learned so much from them, and again, the relatives whom I knew as a kid, who survived World War II. My father was in a destroyer during the D-day invasion, Mary’s father was in North Africa and Italian campaigns and we have relatives who were at Iwo Jima. So again, it’s a wholly different perspective than the average today, I’m from a very different generation. The other thing is I’m very Catholic, it’s what keeps me grounded, where a lot of my inspiration comes from. The biggest, of course, is Mary. Throughout all these twists and turns, Mary’s insight and advice have been the major factor in why we are where we are.
And if there was a very important moment in your career that you’re particularly proud of? I looked through your record and your bio and there are so many high points that I thought it would take probably fifteen minutes just to list all of them. Go ahead if there’s one that just really stands out, that’s very meaningful to you. Of your work, your discoveries, things that you’ve contributed to the knowledge base, or if an award was the most meaningful thing to you, that would be fine, too.
Looking back, two distinct, big things stand out: our pioneering laboratory work on cosmic ice chemistry and on astronomical PAHs. Both have now grown into robust fields of their own. I loved the work and am very grateful for the awards I have received along the way. Among these, I particularly treasure the American Astronomical Society’s inaugural Laboratory Astrophysics Prize. It captures my whole career; it is recognition by my colleagues and one of the great American scientific societies. As for moments, there were so very many, I’ve been blessed. Through no long-term planning of my own, I was in the right place at the right time with the right training over and over and over again! I still use my college chemistry and spectroscopy books from Jim Pegolotti’s Organic Chemistry class in 1966 to interpret spectra. Once NASA’s James Webb Space Telescope starts returning spectra early next year, that book will come out and big moments will soon follow. How fortunate is that?
Did you ever think about being something else besides a scientist?
Oh, that’s an easy one: I wanted to be a cowboy living about a century and a half ago! (laughs) and you can quote me!
OK, we will! Thank you this has been just delightful!
You’re welcome, this has been so much fun!
Interview conducted by Fred and Sara on 7/27/20